A Novel CXCR4 antagonist enhances angiogenesis via modifying the ischaemic tissue environment
- PMID: 28374486
- PMCID: PMC5618675
- DOI: 10.1111/jcmm.13150
A Novel CXCR4 antagonist enhances angiogenesis via modifying the ischaemic tissue environment
Abstract
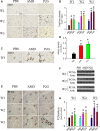
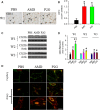
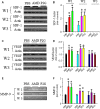
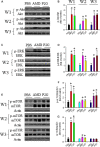
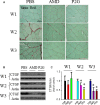
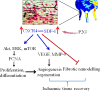
Endothelial progenitor cells (EPCs) play a capital role in angiogenesis via directly participating in neo-vessel formation and secreting pro-angiogenic factors. Stromal cell-derived factor 1 (SDF-1) and its receptor CXCR4 play a critical role in the retention and quiescence of EPCs within its niche in the bone marrow. Disturbing the interaction between SDF-1 and CXCR4 is an effective strategy for EPC mobilization. We developed a novel CXCR4 antagonist P2G, a mutant protein of SDF-1β with high antagonistic activity against CXCR4 and high potency in enhancing ischaemic angiogenesis and blood perfusion. However, its direct effects on ischaemic tissue remain largely unknown. In this study, P2G was found to possess a robust capability to promote EPC infiltration and incorporation in neo-vessels, enhance the expression and function of pro-angiogenic factors, such as SDF-1, vascular endothelial growth factor and matrix metalloprotein-9, and activate cell signals involved in angiogenesis, such as proliferating cell nuclear antigen, protein kinase B (Akt), extracellular regulated protein kinases and mammalian target of rapamycin, in ischaemic tissue. Moreover, P2G can attenuate fibrotic remodelling to facilitate the recovery of ischaemic tissue. The capability of P2G in direct augmenting ischaemic environment for angiogenesis suggests that it is a potential candidate for the therapy of ischaemia diseases.
Keywords: AMD3100; P2G; angiogenesis; endothelial progenitor cells.
© 2017 The Authors. Journal of Cellular and Molecular Medicine published by John Wiley & Sons Ltd and Foundation for Cellular and Molecular Medicine.
Figures
References
-
- Asahara T, Murohara T, Sullivan A, et al Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997; 275: 964–7. - PubMed
-
- Zhao YD, Courtman DW, Deng Y, et al Rescue of monocrotaline‐induced pulmonary arterial hypertension using bone marrow‐derived endothelial‐like progenitor cells: efficacy of combined cell and eNOS gene therapy in established disease. Circ Res. 2005; 96: 442. - PubMed
-
- Sainz J, Sata M. CXCR4, a key modulator of vascular progenitor cells. Arterioscl Throm Vas. 2007; 27: 263. - PubMed
-
- Shantsila E, Watson T, Lip GYH. Endothelial progenitor cells in cardiovascular disorders. J Am Coll Cardiol. 2007; 49: 741–52. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

