Human seizures couple across spatial scales through travelling wave dynamics
- PMID: 28374740
- PMCID: PMC5382286
- DOI: 10.1038/ncomms14896
Human seizures couple across spatial scales through travelling wave dynamics
Abstract
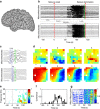
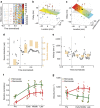
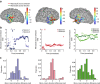
Epilepsy-the propensity toward recurrent, unprovoked seizures-is a devastating disease affecting 65 million people worldwide. Understanding and treating this disease remains a challenge, as seizures manifest through mechanisms and features that span spatial and temporal scales. Here we address this challenge through the analysis and modelling of human brain voltage activity recorded simultaneously across microscopic and macroscopic spatial scales. We show that during seizure large-scale neural populations spanning centimetres of cortex coordinate with small neural groups spanning cortical columns, and provide evidence that rapidly propagating waves of activity underlie this increased inter-scale coupling. We develop a corresponding computational model to propose specific mechanisms-namely, the effects of an increased extracellular potassium concentration diffusing in space-that support the observed spatiotemporal dynamics. Understanding the multi-scale, spatiotemporal dynamics of human seizures-and connecting these dynamics to specific biological mechanisms-promises new insights to treat this devastating disease.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



References
-
- Steinlein O. K., Kaneko S & Hirose S. in Jasper's Basic Mechanisms of the Epilepsies (National Center for Biotechnology Information (US), 2012). - PubMed
-
- Schomer, D. L. & Lopes da Silva, F. H. (eds) Niedermeyer's Electroencephalography: Basic Principles, Clinical Applications, and Related Fields. (Lippincott Williams & Wilkins, 2010).
-
- Gibbs F. A., Gibbs E. L. & Lennox W. G. Epilepsy: a paroxysmal cerebral dysrhythmia. Epilepsy Behav. EB 3, 395–401 (2002). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

