Sialylation Is Dispensable for Early Murine Embryonic Development in Vitro
- PMID: 28374933
- PMCID: PMC5502888
- DOI: 10.1002/cbic.201700083
Sialylation Is Dispensable for Early Murine Embryonic Development in Vitro
Abstract
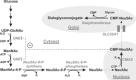
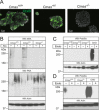
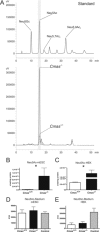
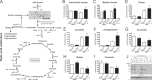
The negatively charged nonulose sialic acid (Sia) is essential for murine development in vivo. In order to elucidate the impact of sialylation on differentiation processes in the absence of maternal influences, we generated mouse embryonic stem cell (mESC) lines that lack CMP-Sia synthetase (CMAS) and thereby the ability to activate Sia to CMP-Sia. Loss of CMAS activity resulted in an asialo cell surface accompanied by an increase in glycoconjugates with terminal galactosyl and oligo-LacNAc residues, as well as intracellular accumulation of free Sia. Remarkably, these changes did not impact intracellular metabolites or the morphology and transcriptome of pluripotent mESC lines. Moreover, the capacity of Cmas-/- mESCs for undirected differentiation into embryoid bodies, germ layer formation and even the generation of beating cardiomyocytes provides first and conclusive evidence that pluripotency and differentiation of mESC in vitro can proceed in the absence of (poly)sialoglycans.
Keywords: CMP-sialic acid synthase; differentiation; glycosylation; metabolism; sialic acids.
© 2017 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

