Heteroatom-Heteroatom Bond Formation in Natural Product Biosynthesis
- PMID: 28375000
- PMCID: PMC5534343
- DOI: 10.1021/acs.chemrev.6b00621
Heteroatom-Heteroatom Bond Formation in Natural Product Biosynthesis
Abstract
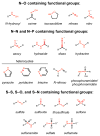
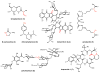
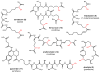
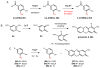
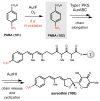
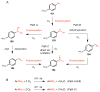
Natural products that contain functional groups with heteroatom-heteroatom linkages (X-X, where X = N, O, S, and P) are a small yet intriguing group of metabolites. The reactivity and diversity of these structural motifs has captured the interest of synthetic and biological chemists alike. Functional groups containing X-X bonds are found in all major classes of natural products and often impart significant biological activity. This review presents our current understanding of the biosynthetic logic and enzymatic chemistry involved in the construction of X-X bond containing functional groups within natural products. Elucidating and characterizing biosynthetic pathways that generate X-X bonds could both provide tools for biocatalysis and synthetic biology, as well as guide efforts to uncover new natural products containing these structural features.
Conflict of interest statement
Notes
The authors declare no competing financial interests.
Figures
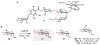





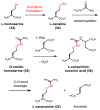



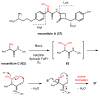
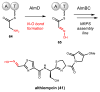

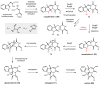
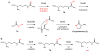












References
-
- Singaram S, Lawrence RS, Hornemann U. Studies on the Biosynthesis of the Antibiotic Streptozotocin (Streptozocin) by Streptomyces achromogenes var. streptozoticus. Feeding Experiments with Carbon-14 and Tritium Labelled Precursors. J Antibiot. 1979;32:379–385. - PubMed
-
- Westlake DWS. Biosynthesis of Chloramphenicol. Biotech Bioeng. 1969;11:1125–1134. - PubMed
-
- Walker S, Gange D, Gupta V, Kahne D. Analysis of Hydroxylamine Glycosidic Linkages: Structural Consequences of the NO Bond in Calicheamicin. J Am Chem Soc. 1994;116:3197–3206.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

