Type 2 innate lymphoid cells treat and prevent acute gastrointestinal graft-versus-host disease
- PMID: 28375154
- PMCID: PMC5409787
- DOI: 10.1172/JCI91816
Type 2 innate lymphoid cells treat and prevent acute gastrointestinal graft-versus-host disease
Abstract
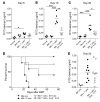
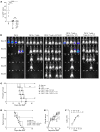
Acute graft-versus-host disease (aGVHD) is the most common complication for patients undergoing allogeneic stem cell transplantation. Despite extremely aggressive therapy targeting donor T cells, patients with grade III or greater aGVHD of the lower GI tract, who do not respond to therapy with corticosteroids, have a dismal prognosis. Thus, efforts to improve understanding of the function of local immune and non-immune cells in regulating the inflammatory process in the GI tract during aGVHD are needed. Here, we demonstrate, using murine models of allogeneic BMT, that type 2 innate lymphoid cells (ILC2s) in the lower GI tract are sensitive to conditioning therapy and show very limited ability to repopulate from donor bone marrow. Infusion of donor ILC2s was effective in reducing the lethality of aGVHD and in treating lower GI tract disease. ILC2 infusion was associated with reduced donor proinflammatory Th1 and Th17 cells, accumulation of donor myeloid-derived suppressor cells (MDSCs) mediated by ILC2 production of IL-13, improved GI tract barrier function, and a preserved graft-versus-leukemia (GVL) response. Collectively, these findings suggest that infusion of donor ILC2s to restore gastrointestinal tract homeostasis may improve treatment of severe lower GI tract aGVHD.
Conflict of interest statement
Figures






Similar articles
-
Type 2 innate lymphoid cells from Id1 transgenic mice alleviate skin manifestations of graft-versus-host disease.BMC Immunol. 2021 Jul 13;22(1):46. doi: 10.1186/s12865-021-00432-w. BMC Immunol. 2021. PMID: 34256699 Free PMC article.
-
Third-party type 2 innate lymphoid cells prevent and treat GI tract GvHD.Blood Adv. 2021 Nov 23;5(22):4578-4589. doi: 10.1182/bloodadvances.2020001514. Blood Adv. 2021. PMID: 34619767 Free PMC article.
-
Efficient treatment of murine acute GvHD by in vitro expanded donor regulatory T cells.Leukemia. 2020 Mar;34(3):895-908. doi: 10.1038/s41375-019-0625-3. Epub 2019 Nov 12. Leukemia. 2020. PMID: 31719679 Free PMC article.
-
Altered homeostatic regulation of innate and adaptive immunity in lower gastrointestinal tract GVHD pathogenesis.J Clin Invest. 2017 Jun 30;127(7):2441-2451. doi: 10.1172/JCI90592. Epub 2017 Jun 5. J Clin Invest. 2017. PMID: 28581444 Free PMC article. Review.
-
Prevention and treatment of acute graft-vs.-host disease.Am J Pediatr Hematol Oncol. 1993 Feb;15(1):28-48. doi: 10.1097/00043426-199302000-00004. Am J Pediatr Hematol Oncol. 1993. PMID: 8383475 Review.
Cited by
-
Role of myeloid-derived suppressor cells in the promotion and immunotherapy of colitis-associated cancer.J Immunother Cancer. 2020 Oct;8(2):e000609. doi: 10.1136/jitc-2020-000609. J Immunother Cancer. 2020. PMID: 33051339 Free PMC article. Review.
-
Strategies for Enhancing and Preserving Anti-leukemia Effects Without Aggravating Graft-Versus-Host Disease.Front Immunol. 2018 Dec 21;9:3041. doi: 10.3389/fimmu.2018.03041. eCollection 2018. Front Immunol. 2018. PMID: 30619371 Free PMC article. Review.
-
Acute GVHD: think before you treat.Hematology Am Soc Hematol Educ Program. 2021 Dec 10;2021(1):642-647. doi: 10.1182/hematology.2021000300. Hematology Am Soc Hematol Educ Program. 2021. PMID: 34889409 Free PMC article. Review.
-
Novel Insights Into the Mechanism of GVHD-Induced Tissue Damage.Front Immunol. 2021 Aug 27;12:713631. doi: 10.3389/fimmu.2021.713631. eCollection 2021. Front Immunol. 2021. PMID: 34512636 Free PMC article. Review.
-
Innate immune cellular therapeutics in transplantation.Front Transplant. 2023;2:1067512. doi: 10.3389/frtra.2023.1067512. Epub 2023 Mar 31. Front Transplant. 2023. PMID: 37994308 Free PMC article.
References
-
- Faber EA, Vose JM. The role of hematopoietic stem cell transplant in follicular lymphoma. J Natl Compr Canc Netw. 2010;8(3):337–344. - PubMed
MeSH terms
Grants and funding
- P01 CA065493/CA/NCI NIH HHS/United States
- R01 HL115761/HL/NHLBI NIH HHS/United States
- P30 CA016086/CA/NCI NIH HHS/United States
- R37 AI034495/AI/NIAID NIH HHS/United States
- MC_U105178805/MRC_/Medical Research Council/United Kingdom
- F32 HL126365/HL/NHLBI NIH HHS/United States
- R01 HL139730/HL/NHLBI NIH HHS/United States
- R01 HL056067/HL/NHLBI NIH HHS/United States
- WT_/Wellcome Trust/United Kingdom
- R01 CA072669/CA/NCI NIH HHS/United States
- R37 AI029564/AI/NIAID NIH HHS/United States
- MR/M011755/1/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

