Megakaryocytes compensate for Kit insufficiency in murine arthritis
- PMID: 28375155
- PMCID: PMC5409069
- DOI: 10.1172/JCI84598
Megakaryocytes compensate for Kit insufficiency in murine arthritis
Abstract
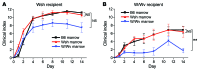
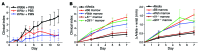
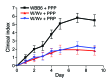
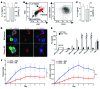
The growth factor receptor Kit is involved in hematopoietic and nonhematopoietic development. Mice bearing Kit defects lack mast cells; however, strains bearing different Kit alleles exhibit diverse phenotypes. Herein, we investigated factors underlying differential sensitivity to IgG-mediated arthritis in 2 mast cell-deficient murine lines: KitWsh/Wsh, which develops robust arthritis, and KitW/Wv, which does not. Reciprocal bone marrow transplantation between KitW/Wv and KitWsh/Wsh mice revealed that arthritis resistance reflects a hematopoietic defect in addition to mast cell deficiency. In KitW/Wv mice, restoration of susceptibility to IgG-mediated arthritis was neutrophil independent but required IL-1 and the platelet/megakaryocyte markers NF-E2 and glycoprotein VI. In KitW/Wv mice, platelets were present in numbers similar to those in WT animals and functionally intact, and transfer of WT platelets did not restore arthritis susceptibility. These data implicated a platelet-independent role for the megakaryocyte, a Kit-dependent lineage that is selectively deficient in KitW/Wv mice. Megakaryocytes secreted IL-1 directly and as a component of circulating microparticles, which activated synovial fibroblasts in an IL-1-dependent manner. Transfer of WT but not IL-1-deficient megakaryocytes restored arthritis susceptibility to KitW/Wv mice. These findings identify functional redundancy among Kit-dependent hematopoietic lineages and establish an unanticipated capacity of megakaryocytes to mediate IL-1-driven systemic inflammatory disease.
Conflict of interest statement
Figures


References
-
- Ehrlich P. Beiträge zur Theorie und Praxis der histologischen Färbung [doctoral thesis]. Leipzig, Germany: Leipzig University; 1878.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

