ω-3 polyunsaturated fatty acids ameliorate type 1 diabetes and autoimmunity
- PMID: 28375156
- PMCID: PMC5409789
- DOI: 10.1172/JCI87388
ω-3 polyunsaturated fatty acids ameliorate type 1 diabetes and autoimmunity
Abstract
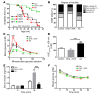
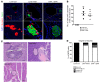
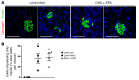
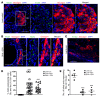
Despite the benefit of insulin, blockade of autoimmune attack and regeneration of pancreatic islets are ultimate goals for the complete cure of type 1 diabetes (T1D). Long-term consumption of ω-3 polyunsaturated fatty acids (PUFAs) is known to suppress inflammatory processes, making these fatty acids candidates for the prevention and amelioration of autoimmune diseases. Here, we explored the preventative and therapeutic effects of ω-3 PUFAs on T1D. In NOD mice, dietary intervention with ω-3 PUFAs sharply reduced the incidence of T1D, modulated the differentiation of Th cells and Tregs, and decreased the levels of IFN-γ, IL-17, IL-6, and TNF-α. ω-3 PUFAs exerted similar effects on the differentiation of CD4+ T cells isolated from human peripheral blood mononuclear cells. The regulation of CD4+ T cell differentiation was mediated at least in part through ω-3 PUFA eicosanoid derivatives and by mTOR complex 1 (mTORC1) inhibition. Importantly, therapeutic intervention in NOD mice through nutritional supplementation or lentivirus-mediated expression of an ω-3 fatty acid desaturase, mfat-1, normalized blood glucose and insulin levels for at least 182 days, blocked the development of autoimmunity, prevented lymphocyte infiltration into regenerated islets, and sharply elevated the expression of the β cell markers pancreatic and duodenal homeobox 1 (Pdx1) and paired box 4 (Pax4). The findings suggest that ω-3 PUFAs could potentially serve as a therapeutic modality for T1D.
Conflict of interest statement
Figures





Similar articles
-
Self-Transducible Bimodal PDX1-FOXP3 Protein Lifts Insulin Secretion and Curbs Autoimmunity, Boosting Tregs in Type 1 Diabetic Mice.Mol Ther. 2018 Jan 3;26(1):184-198. doi: 10.1016/j.ymthe.2017.08.014. Epub 2017 Sep 7. Mol Ther. 2018. PMID: 28988715 Free PMC article.
-
Pancreatic duodenal homeobox 1 protein is a novel beta-cell-specific autoantigen for type I diabetes.Lab Invest. 2010 Jan;90(1):31-9. doi: 10.1038/labinvest.2009.116. Epub 2009 Nov 9. Lab Invest. 2010. PMID: 19901909 Free PMC article.
-
Oral administration of PDX1 confers protection against insulitis in the non-obese diabetic (NOD) mice.Biochem Biophys Res Commun. 2015 Oct 30;466(4):656-63. doi: 10.1016/j.bbrc.2015.09.098. Epub 2015 Sep 21. Biochem Biophys Res Commun. 2015. PMID: 26403969
-
Vitamin D and Omega-3 Polyunsaturated Fatty Acids in Type 1 Diabetes Modulation.Endocr Metab Immune Disord Drug Targets. 2022;22(8):815-833. doi: 10.2174/1871530322666220103114450. Endocr Metab Immune Disord Drug Targets. 2022. PMID: 34979894 Review.
-
Control of type 1 autoimmune diabetes by naturally occurring CD4+CD25+ regulatory T lymphocytes in neonatal NOD mice.Ann N Y Acad Sci. 2005 Jun;1051:72-87. doi: 10.1196/annals.1361.048. Ann N Y Acad Sci. 2005. PMID: 16126946 Review.
Cited by
-
Lipid metabolism in type 1 diabetes mellitus: Pathogenetic and therapeutic implications.Front Immunol. 2022 Oct 6;13:999108. doi: 10.3389/fimmu.2022.999108. eCollection 2022. Front Immunol. 2022. PMID: 36275658 Free PMC article. Review.
-
The gut microbiota and endometriosis: From pathogenesis to diagnosis and treatment.Front Cell Infect Microbiol. 2022 Nov 24;12:1069557. doi: 10.3389/fcimb.2022.1069557. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36506023 Free PMC article. Review.
-
Redox Remodeling by Nutraceuticals for Prevention and Treatment of Acute and Chronic Inflammation.Antioxidants (Basel). 2023 Jan 5;12(1):132. doi: 10.3390/antiox12010132. Antioxidants (Basel). 2023. PMID: 36670995 Free PMC article. Review.
-
Metabolic and Metabolomic Insights Regarding the Omega-3 PUFAs Intake in Type 1 Diabetes Mellitus.Front Mol Biosci. 2021 Dec 3;8:783065. doi: 10.3389/fmolb.2021.783065. eCollection 2021. Front Mol Biosci. 2021. PMID: 34926582 Free PMC article. Review.
-
EPA Induces an Anti-Inflammatory Transcriptome in T Cells, Implicating a Triglyceride-Independent Pathway in Cardiovascular Risk Reduction.JACC Basic Transl Sci. 2025 Mar;10(3):383-395. doi: 10.1016/j.jacbts.2024.09.002. Epub 2024 Oct 30. JACC Basic Transl Sci. 2025. PMID: 40139879 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

