Isolated polycystic liver disease genes define effectors of polycystin-1 function
- PMID: 28375157
- PMCID: PMC5409105
- DOI: 10.1172/JCI90129
Isolated polycystic liver disease genes define effectors of polycystin-1 function
Erratum in
-
Isolated polycystic liver disease genes define effectors of polycystin-1 function.J Clin Invest. 2017 Sep 1;127(9):3558. doi: 10.1172/JCI96729. Epub 2017 Sep 1. J Clin Invest. 2017. PMID: 28862642 Free PMC article.
Abstract
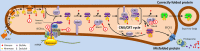
Dominantly inherited isolated polycystic liver disease (PCLD) consists of liver cysts that are radiologically and pathologically identical to those seen in autosomal dominant polycystic kidney disease, but without clinically relevant kidney cysts. The causative genes are known for fewer than 40% of PCLD index cases. Here, we have used whole exome sequencing in a discovery cohort of 102 unrelated patients who were excluded for mutations in the 2 most common PCLD genes, PRKCSH and SEC63, to identify heterozygous loss-of-function mutations in 3 additional genes, ALG8, GANAB, and SEC61B. Similarly to PRKCSH and SEC63, these genes encode proteins that are integral to the protein biogenesis pathway in the endoplasmic reticulum. We inactivated these candidate genes in cell line models to show that loss of function of each results in defective maturation and trafficking of polycystin-1, the central determinant of cyst pathogenesis. Despite acting in a common pathway, each PCLD gene product demonstrated distinct effects on polycystin-1 biogenesis. We also found enrichment on a genome-wide basis of heterozygous mutations in the autosomal recessive polycystic kidney disease gene PKHD1, indicating that adult PKHD1 carriers can present with clinical PCLD. These findings define genetic and biochemical modulators of polycystin-1 function and provide a more complete definition of the spectrum of dominant human polycystic diseases.
Conflict of interest statement
Figures

Comment in
-
Genetics: Novel causative genes for polycystic liver disease.Nat Rev Gastroenterol Hepatol. 2017 Jul;14(7):391-392. doi: 10.1038/nrgastro.2017.69. Epub 2017 May 31. Nat Rev Gastroenterol Hepatol. 2017. PMID: 28559591 No abstract available.
-
Polycystic liver disease: The interplay of genes causative for hepatic and renal cystogenesis.Hepatology. 2018 Jun;67(6):2462-2464. doi: 10.1002/hep.29708. Epub 2018 Apr 19. Hepatology. 2018. PMID: 29211938 Free PMC article. No abstract available.
References
-
- Desmet VJ. Ludwig symposium on biliary disorders — part I. Pathogenesis of ductal plate abnormalities. Mayo Clin Proc. 1998;73(1):80–89. - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
- R01 DK051041/DK/NIDDK NIH HHS/United States
- P30 DK090868/DK/NIDDK NIH HHS/United States
- R01 DK054053/DK/NIDDK NIH HHS/United States
- UL1 TR001863/TR/NCATS NIH HHS/United States
- P30 DK079310/DK/NIDDK NIH HHS/United States
- T32 DK007276/DK/NIDDK NIH HHS/United States
- U54 HG006504/HG/NHGRI NIH HHS/United States
- R01 DK100592/DK/NIDDK NIH HHS/United States
- R01 DK044863/DK/NIDDK NIH HHS/United States
- R01 DK103184/DK/NIDDK NIH HHS/United States
- UM1 HG006504/HG/NHGRI NIH HHS/United States
- P30 DK090728/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

