Dual-Phase Iontophoresis for the Delivery of Antisense Oligonucleotides
- PMID: 28375679
- PMCID: PMC5564040
- DOI: 10.1089/nat.2016.0654
Dual-Phase Iontophoresis for the Delivery of Antisense Oligonucleotides
Abstract
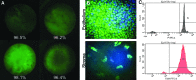
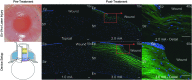
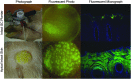
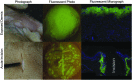
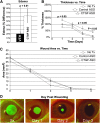
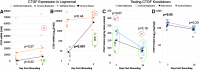
In support of ongoing research in the study of corneal and skin wound healing, we sought to improve on previously published results by using iontophoresis to deliver RNA interference-based oligonucleotides. By using a electromechanics-based approach, we were able to devise a two-phase solution that separated the buffering solution from the antisense oligonucleotide (ASO) solution. The separation was obtained by making the drug solution a higher density than the buffer, leading it to sink directly onto the tissue surface. This change immediately decreased the distance that the ASO would have to travel before delivery. The changes enabled delivery into ex vivo skin and corneas in 10 or fewer minutes and into in vivo corneas in 5 min. In vivo studies demonstrated short-term bioavailability of at least 24 h, a lack of chemical or thermal injury, a lack of interference in the healing of a corneal injury, and an antisense effect till at least day 7, but not day 14. The only side-effect observed was postdelivery edema that was not present when the vehicle alone was iontophoresed. This suggests that electro-osmotic flow from the delivery chamber was not the mechanism, but that the delivered solute likely increased the tissue's osmolarity. These results support the continued development and utilization of this ASO delivery approach in research-grade oligonucleotides to probe molecular biological pathways and in support of testing therapeutic ASOs in the skin and cornea.
Keywords: antisense; biological variance; cornea; delivery; iontophoresis; skin.
Conflict of interest statement
D.J.G.: Patent: U.S. 8,838,229, issued: yes, licensed: no, royalties: no, Grant: Trainee on a NEI T32 Vision Training grant (T32-EY007132). S.S.T.: Patent: U.S. 8,838,229, issued: yes, licensed: no, royalties: no, Grant: Unrestricted grant from Research to Prevent Blindness. G.S.S.: Patent: U.S. 8,838,229, issued: yes, licensed: no, royalties: no, Grant: PI on a R01 Regulation of Stromal Wound Healing (R01-EY05587). All three authors are co-inventors on a patent (U.S. 8,838,229), which has been issued, but has neither been licensed nor generated any royalties.
Figures








References
-
- Sisco M, Kryger ZB, O'Shaughnessy KD, Kim PS, Schultz GS, Ding XZ, Roy NK, Dean NM. and Mustoe TA. (2008). Antisense inhibition of connective tissue growth factor (CTGF/CCN2) mRNA limits hypertrophic scarring without affecting wound healing in vivo. Wound Repair Regen 16:661–673 - PubMed
-
- Rae JL. and Blankenship JE. (1973). Bioelectric measurements in the frog lens. Exp Eye Res 15:209–217 - PubMed
-
- Araie M. and Maurice DM. (1985). A reevaluation of corneal endothelial permeability to fluorescein. Exp Eye Res 41:383–390 - PubMed
-
- Koretz JF, Bertasso AM, Neider MW, True-Gabelt BA. and Kaufman PL. (1987). Slit-lamp studies of the rhesus monkey eye: II. Changes in crystalline lens shape, thickness and position during accommodation and aging. Exp Eye Res 45:317–326 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

