Mammographic Density Change With Estrogen and Progestin Therapy and Breast Cancer Risk
- PMID: 28376149
- PMCID: PMC6059178
- DOI: 10.1093/jnci/djx001
Mammographic Density Change With Estrogen and Progestin Therapy and Breast Cancer Risk
Abstract
Background: Estrogen plus progestin therapy increases both mammographic density and breast cancer incidence. Whether mammographic density change associated with estrogen plus progestin initiation predicts breast cancer risk is unknown.
Methods: We conducted an ancillary nested case-control study within the Women's Health Initiative trial that randomly assigned postmenopausal women to daily conjugated equine estrogen 0.625 mg plus medroxyprogesterone acetate 2.5 mg or placebo. Mammographic density was assessed from mammograms taken prior to and one year after random assignment for 174 women who later developed breast cancer (cases) and 733 healthy women (controls). Logistic regression analyses included adjustment for confounders and baseline mammographic density when appropriate.
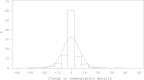
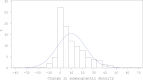
Results: Among women in the estrogen plus progestin arm (97 cases/378 controls), each 1% positive change in percent mammographic density increased breast cancer risk 3% (odds ratio [OR] = 1.03, 95% confidence interval [CI] = 1.01 to 1.06). For women in the highest quintile of mammographic density change (>19.3% increase), breast cancer risk increased 3.6-fold (95% CI = 1.52 to 8.56). The effect of estrogen plus progestin use on breast cancer risk (OR = 1.28, 95% CI = 0.90 to 1.82) was eliminated in this study, after adjusting for change in mammographic density (OR = 1.00, 95% CI = 0.66 to 1.51).
Conclusions: We found the one-year change in mammographic density after estrogen plus progestin initiation predicted subsequent increase in breast cancer risk. All of the increased risk from estrogen plus progestin use was mediated through mammographic density change. Doctors should evaluate changes in mammographic density with women who initiate estrogen plus progestin therapy and discuss the breast cancer risk implications.
Published by Oxford University Press 2017. This work is written by US Government employees and is in the public domain in the US.
Figures
Similar articles
-
Estrogen-plus-progestin use and mammographic density in postmenopausal women: Women's Health Initiative randomized trial.J Natl Cancer Inst. 2005 Sep 21;97(18):1366-76. doi: 10.1093/jnci/dji279. J Natl Cancer Inst. 2005. PMID: 16174858 Clinical Trial.
-
Postmenopausal hormone therapy and change in mammographic density.J Natl Cancer Inst. 2003 Jan 1;95(1):30-7. doi: 10.1093/jnci/95.1.30. J Natl Cancer Inst. 2003. PMID: 12509398
-
Effects of estrogen and estrogen-progestin on mammographic parenchymal density. Postmenopausal Estrogen/Progestin Interventions (PEPI) Investigators.Ann Intern Med. 1999 Feb 16;130(4 Pt 1):262-9. doi: 10.7326/0003-4819-130-4_part_1-199902160-00003. Ann Intern Med. 1999. PMID: 10068383 Clinical Trial.
-
The Women's Health Initiative trial and related studies: 10 years later: a clinician's view.J Steroid Biochem Mol Biol. 2014 Jul;142:4-11. doi: 10.1016/j.jsbmb.2013.10.009. Epub 2013 Oct 27. J Steroid Biochem Mol Biol. 2014. PMID: 24172877 Review.
-
New evidence regarding hormone replacement therapies is urgently required transdermal postmenopausal hormone therapy differs from oral hormone therapy in risks and benefits.Maturitas. 2005 Sep 16;52(1):1-10. doi: 10.1016/j.maturitas.2005.05.003. Maturitas. 2005. PMID: 15963666 Review.
Cited by
-
Environmental Influences on Mammographic Breast Density in California: A Strategy to Reduce Breast Cancer Risk.Int J Environ Res Public Health. 2019 Nov 27;16(23):4731. doi: 10.3390/ijerph16234731. Int J Environ Res Public Health. 2019. PMID: 31783496 Free PMC article.
-
Serum concentrations of DDE, PCBs, and other persistent organic pollutants and mammographic breast density in Triana, Alabama, a highly exposed population.Environ Res. 2020 Mar;182:109068. doi: 10.1016/j.envres.2019.109068. Epub 2019 Dec 26. Environ Res. 2020. PMID: 31918312 Free PMC article.
-
The origins of breast cancer associated with mammographic density: a testable biological hypothesis.Breast Cancer Res. 2018 Mar 7;20(1):17. doi: 10.1186/s13058-018-0941-y. Breast Cancer Res. 2018. PMID: 29514672 Free PMC article.
-
Association between menopausal hormone therapy, mammographic density and breast cancer risk: results from the E3N cohort study.Breast Cancer Res. 2021 Apr 17;23(1):47. doi: 10.1186/s13058-021-01425-8. Breast Cancer Res. 2021. PMID: 33865453 Free PMC article.
-
The Role of Gut Microbial β-Glucuronidase in Estrogen Reactivation and Breast Cancer.Front Cell Dev Biol. 2021 Aug 12;9:631552. doi: 10.3389/fcell.2021.631552. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34458248 Free PMC article. Review.
References
-
- Rossouw JE, Anderson GL, Prentice RL, et al.Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results from the Women's Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–333. - PubMed
-
- Chlebowski RT, Hendrix SL, Langer RD, et al.Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women: The Women's Health Initiative Randomized Trial. JAMA. 2003;289(24):3243–3253. - PubMed
-
- Hersh AL, Stefanick ML, Stafford RS.. National use of postmenopausal hormone therapy: Annual trends and response to recent evidence. JAMA. 2004;291(1):47–53. - PubMed
-
- Haas JS, Kaplan CP, Gerstenberger EP, Kerlikowske K.. Changes in the use of postmenopausal hormone therapy after the publication of clinical trial results. Ann Intern Med. 2004;140(3):184–188. - PubMed
-
- Hing E, Brett KM.. Changes in US prescribing patterns of menopausal hormone therapy, 2001–2003. Obstet Gynecol. 2006;108(1):33–40. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

