Mammographic Density Change With Estrogen and Progestin Therapy and Breast Cancer Risk
- PMID: 28376149
- PMCID: PMC6059178
- DOI: 10.1093/jnci/djx001
Mammographic Density Change With Estrogen and Progestin Therapy and Breast Cancer Risk
Abstract
Background: Estrogen plus progestin therapy increases both mammographic density and breast cancer incidence. Whether mammographic density change associated with estrogen plus progestin initiation predicts breast cancer risk is unknown.
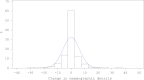
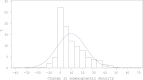
Methods: We conducted an ancillary nested case-control study within the Women's Health Initiative trial that randomly assigned postmenopausal women to daily conjugated equine estrogen 0.625 mg plus medroxyprogesterone acetate 2.5 mg or placebo. Mammographic density was assessed from mammograms taken prior to and one year after random assignment for 174 women who later developed breast cancer (cases) and 733 healthy women (controls). Logistic regression analyses included adjustment for confounders and baseline mammographic density when appropriate.
Results: Among women in the estrogen plus progestin arm (97 cases/378 controls), each 1% positive change in percent mammographic density increased breast cancer risk 3% (odds ratio [OR] = 1.03, 95% confidence interval [CI] = 1.01 to 1.06). For women in the highest quintile of mammographic density change (>19.3% increase), breast cancer risk increased 3.6-fold (95% CI = 1.52 to 8.56). The effect of estrogen plus progestin use on breast cancer risk (OR = 1.28, 95% CI = 0.90 to 1.82) was eliminated in this study, after adjusting for change in mammographic density (OR = 1.00, 95% CI = 0.66 to 1.51).
Conclusions: We found the one-year change in mammographic density after estrogen plus progestin initiation predicted subsequent increase in breast cancer risk. All of the increased risk from estrogen plus progestin use was mediated through mammographic density change. Doctors should evaluate changes in mammographic density with women who initiate estrogen plus progestin therapy and discuss the breast cancer risk implications.
Published by Oxford University Press 2017. This work is written by US Government employees and is in the public domain in the US.
Figures
References
-
- Rossouw JE, Anderson GL, Prentice RL, et al.Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results from the Women's Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–333. - PubMed
-
- Chlebowski RT, Hendrix SL, Langer RD, et al.Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women: The Women's Health Initiative Randomized Trial. JAMA. 2003;289(24):3243–3253. - PubMed
-
- Hersh AL, Stefanick ML, Stafford RS.. National use of postmenopausal hormone therapy: Annual trends and response to recent evidence. JAMA. 2004;291(1):47–53. - PubMed
-
- Haas JS, Kaplan CP, Gerstenberger EP, Kerlikowske K.. Changes in the use of postmenopausal hormone therapy after the publication of clinical trial results. Ann Intern Med. 2004;140(3):184–188. - PubMed
-
- Hing E, Brett KM.. Changes in US prescribing patterns of menopausal hormone therapy, 2001–2003. Obstet Gynecol. 2006;108(1):33–40. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

