Evidence for a Mesothelial Origin of Body Cavity Effusion Lymphomas
- PMID: 28376153
- PMCID: PMC6059237
- DOI: 10.1093/jnci/djx016
Evidence for a Mesothelial Origin of Body Cavity Effusion Lymphomas
Abstract
Background: Primary effusion lymphoma (PEL) is a Kaposi's sarcoma herpes virus (KSHV)-induced lymphoma that typically arises in body cavities of HIV-infected patients. PEL cells are often co-infected with Epstein-Barr virus (EBV). "PEL-like" lymphoma is a KSHV-unrelated lymphoma that arises in body cavities of HIV-negative patients. "PEL-like" lymphoma is sometimes EBV positive. The derivation of PEL/"PEL-like" cells is unclear.
Methods: Mesothelial cells were cultured from body cavity effusions of 23 patients. Cell proliferation, cytokine secretion, marker phenotypes, KSHV/EBV infection, and clonality were evaluated by standard methods. Gene expression was measured by quantitative polymerase chain reaction and immunoblotting. A mouse model of PEL (3 mice/group) was used to evaluate tumorigenicity.
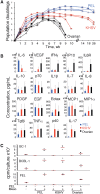
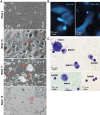
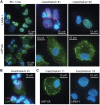
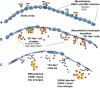
Results: We found that the mesothelia derived from six effusions of HIV-infected patients with PEL or other KSHV-associated diseases contained rare KSHV + or EBV + mesothelial cells. After extended culture (16-17 weeks), some mesothelial cells underwent a trans-differentiation process, generating lymphoid-type CD45 + /B220 + , CD5 + , CD27 + , CD43 + , CD11c + , and CD3 - cells resembling "B1-cells," most commonly found in mouse body cavities. These "B1-like" cells were short lived. However, long-term KSHV + EBV - and EBV + KSHV - clonal cell lines emerged from mesothelial cultures from two patients that were clonally distinct from the monoclonal or polyclonal B-cell populations found in the patients' original effusions.
Conclusions: Mesothelial-to-lymphoid transformation is a newly identified in vitro process that generates "B1-like" cells and is associated with the emergence of long-lived KSHV or EBV-infected cell lines in KSHV-infected patients. These results identify mesothelial cultures as a source of PEL cells and lymphoid cells in humans.
Published by Oxford University Press 2017. This work is written by US Government employees and is in the public domain in the US.
Figures


References
-
- Knowles DM, Inghirami G, Ubriaco A, et al.Molecular genetic analysis of three AIDS-associated neoplasms of uncertain lineage demonstrates their B-cell derivation and the possible pathogenetic role of the Epstein-Barr virus. Blood. 1989;73(3):792–799. - PubMed
-
- Cesarman E, Chang Y, Moore PS, et al.Kaposi's sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N Engl J Med. 1995;332(18):1186–1191. - PubMed
-
- Chadburn A, Hyjek EM, Tam W, et al.Immunophenotypic analysis of the Kaposi sarcoma herpesvirus (KSHV; HHV-8)-infected B cells in HIV+ multicentric Castleman disease (MCD). Histopathology. 2008;53(5):513–524. - PubMed
-
- Ascoli V, Calabro ML, Giannakakis K, et al.Kaposi's sarcoma-associated herpesvirus/human herpesvirus 8-associated polyclonal body cavity effusions that mimic primary effusion lymphomas. Int J Cancer. 2006;119(7):1746–1748; author reply 1749–1750. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

