Role of Antigen Spread and Distinctive Characteristics of Immunotherapy in Cancer Treatment
- PMID: 28376158
- PMCID: PMC5441294
- DOI: 10.1093/jnci/djw261
Role of Antigen Spread and Distinctive Characteristics of Immunotherapy in Cancer Treatment
Abstract
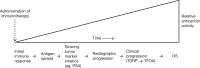
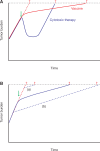
Immunotherapy is an important breakthrough in cancer. US Food and Drug Administration-approved immunotherapies for cancer treatment (including, but not limited to, sipuleucel-T, ipilimumab, nivolumab, pembrolizumab, and atezolizumab) substantially improve overall survival across multiple malignancies. One mechanism of action of these treatments is to induce an immune response against antigen-bearing tumor cells; the resultant cell death releases secondary (nontargeted) tumor antigens. Secondary antigens prime subsequent immune responses (antigen spread). Immunotherapy-induced antigen spread has been shown in clinical studies. For example, in metastatic castration-resistant prostate cancer patients, sipuleucel-T induced early immune responses to the immunizing antigen (PA2024) and/or the target antigen (prostatic acid phosphatase). Thereafter, most patients developed increased antibody responses to numerous secondary proteins, several of which are expressed in prostate cancer with functional relevance in cancer. The ipilimumab-induced antibody profile in melanoma patients shows that antigen spread also occurs with immune checkpoint blockade. In contrast to chemotherapy, immunotherapy often does not result in short-term changes in conventional disease progression end points (eg, progression-free survival, tumor size), which may be explained, in part, by the time taken for antigen spread to occur. Thus, immune-related response criteria need to be identified to better monitor the effectiveness of immunotherapy. As immunotherapy antitumor effects take time to evolve, immunotherapy in patients with less advanced cancer may have greater clinical benefit vs those with more advanced disease. This concept is supported by prostate cancer clinical studies with sipuleucel-T, PSA-TRICOM, and ipilimumab. We discuss antigen spread with cancer immunotherapy and its implications for clinical outcomes.
© The Author 2017. Published by Oxford University Press. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures


References
-
- Science's Top 10 Breakthroughs of 2013. Science AAAS. http://www.sciencemag.org/news/2013/12/sciences-top-10-breakthroughs-2013-0. Accessed July 21, 2016.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

