A Plasma Biomarker Panel to Identify Surgically Resectable Early-Stage Pancreatic Cancer
- PMID: 28376184
- PMCID: PMC6059209
- DOI: 10.1093/jnci/djw341
A Plasma Biomarker Panel to Identify Surgically Resectable Early-Stage Pancreatic Cancer
Abstract
Background: Blood-based biomarkers for early detection of pancreatic ductal adenocarcinoma (PDAC) are urgently needed. Current biomarkers lack high sensitivity and specificity for population screening. The gold-standard biomarker, CA 19-9, also fails to demonstrate the predictive value necessary for early detection.
Methods: To validate a functional genomics-based plasma migration signature biomarker panel, plasma tissue factor pathway inhibitor (TFPI), tenascin C (TNC-FN III-C), and CA 19-9 levels were measured by enzyme-linked immunosorbent assays in three early-stage PDAC plasma cohorts, including two independent blinded validation cohorts containing a total of 43 stage I, 163 stage II, 86 chronic pancreatitis, 31 acute biliary obstruction, and 108 controls. Logistic regression models developed classification rules combining TFPI and/or TNC-FN III-C with CA 19-9 for patient cases and control subjects, with or without adjustment for age and diabetes status. Model classification performance was evaluated and analyses repeated among subpopulations without diabetes and pancreatitis history. Two-sided P values were calculated using bootstrap method.
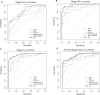
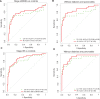
Results: The TFPI/TNC-FN III-C/CA 19-9 panel improved CA 19-9 performance in all early-stage cohorts, including discriminating stage IA/IB/IIA, stage IIB, and all early-stage cancer from healthy controls. Statistical significance was reached for a number of subcohorts, including for all early-stage cancer vs healthy controls (cohort 1 AUC = 0.92, 95% CI = 0.86 to 0.96, P = .04; cohort 3 AUC = 0.83, 95% CI = 0.76 to 0.89, P = .045). Among subcohorts without diabetes and pancreatitis history, the panel approaches potential clinical utility for early detection to discriminate early-stage PDAC from healthy controls including an area under the curve (AUC) of 0.87 (95% CI = 0.77 to 0.95) for stage I/IIA, an AUC of 0.93 (95% CI = 0.87 to 0.98) for stage IIB, and a statistically significant AUC of 0.89 (95% CI = 0.82 to 0.95) for all early-stage cancer ( P = .03).
Conclusions: TFPI/TNC-FN III-C migration signature adds statistically significantly to CA 19-9's predictive power to detect early-stage PDAC and may have clinical utility for early detection of surgically resectable PDAC, as well as for enhanced survival from this routinely lethal cancer.
© The Author 2017. Published by Oxford University Press. All rights reserved. For Permissions, please e-mail: journals.permissions@oup.com.
Figures

Comment in
-
Circulating Biomarkers to Identify Patients With Resectable Pancreatic Cancer.J Natl Cancer Inst. 2017 Aug 1;109(8):djx004. doi: 10.1093/jnci/djx004. J Natl Cancer Inst. 2017. PMID: 28376185 Free PMC article. No abstract available.
References
-
- Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the united states. Cancer Res. 2014;74:2913–2921. - PubMed
-
- Chu D, Kohlmann W, Adler DG. Identification and screening of individuals at increased risk for pancreatic cancer with emphasis on known environmental and genetic factors and hereditary syndromes. JOP. 2010;11:203–212. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

