Kinetics of the Human Papillomavirus Type 16 E6 Antibody Response Prior to Oropharyngeal Cancer
- PMID: 28376197
- PMCID: PMC5967352
- DOI: 10.1093/jnci/djx005
Kinetics of the Human Papillomavirus Type 16 E6 Antibody Response Prior to Oropharyngeal Cancer
Abstract
Background: In a European cohort, it was previously reported that 35% of oropharyngeal cancer (OPC) patients were human papillomavirus type-16 (HPV16) seropositive up to 10 years before diagnosis vs 0.6% of cancer-free controls. Here, we describe the kinetics of HPV16-E6 antibodies prior to OPC diagnosis.
Methods: We used annual serial prediagnostic blood samples from the PLCO Cancer Screening Trial. Antibodies to HPV were initially assessed in prediagnostic blood drawn at study enrollment from 198 incident head and neck cancer patients (median years to cancer diagnosis = 6.6) and 924 matched control subjects using multiplex serology, and subsequently in serial samples (median = 5/individual). Available tumor samples were identified and tested for HPV16 RNA to define HPV-driven OPC.
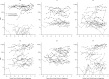
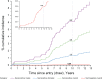
Results: HPV16-E6 antibodies were present at baseline in 42.3% of 52 OPC patients and 0.5% of 924 control subjects. HPV16-E6 antibody levels were highly elevated and stable across serial blood samples for 21 OPC patients who were seropositive at baseline, as well as for one OPC patient who seroconverted closer to diagnosis. All five subjects with HPV16-driven OPC tumors were HPV16-E6-seropositive, and the four subjects with HPV16-negative OPC tumors were seronegative. The estimated 10-year cumulative risk of OPC was 6.2% (95% confidence interval [CI] = 1.8% to 21.5%) for HPV16-E6-seropositive men, 1.3% (95% CI = 0.1% to 15.3%) for HPV16-E6-seropositive women, and 0.04% (95% CI = 0.03% to 0.06%) among HPV16-E6-seronegative individuals.
Conclusions: Forty-two percent of subjects diagnosed with OPC between 1994 and 2009 in a US cohort were HPV16-E6 seropositive, with stable antibody levels during annual follow-up for up to 13 years prior to diagnosis. Tumor analysis indicated that the sensitivity and specificity of HPV16-E6 antibodies were exceptionally high in predicting HPV-driven OPC.
Published by Oxford University Press 2017. This work is written by US Government employees and is in the public domain in the US.
Figures

Similar articles
-
Human papillomavirus 16 E6 antibodies are sensitive for human papillomavirus-driven oropharyngeal cancer and are associated with recurrence.Cancer. 2017 Nov 15;123(22):4382-4390. doi: 10.1002/cncr.30966. Epub 2017 Sep 26. Cancer. 2017. PMID: 28950407 Free PMC article.
-
Natural history of HPV-16 E6 serology among cancer-free men in a multicenter longitudinal cohort study.J Natl Cancer Inst. 2025 May 1;117(5):915-923. doi: 10.1093/jnci/djae326. J Natl Cancer Inst. 2025. PMID: 39658222 Free PMC article.
-
Timing of HPV16-E6 antibody seroconversion before OPSCC: findings from the HPVC3 consortium.Ann Oncol. 2019 Aug 1;30(8):1335-1343. doi: 10.1093/annonc/mdz138. Ann Oncol. 2019. PMID: 31185496 Free PMC article.
-
Sensitivity and specificity of antibodies against HPV16 E6 and other early proteins for the detection of HPV16-driven oropharyngeal squamous cell carcinoma.Int J Cancer. 2017 Jun 15;140(12):2748-2757. doi: 10.1002/ijc.30697. Epub 2017 Apr 4. Int J Cancer. 2017. PMID: 28316084
-
Characterization of human papillomavirus antibodies in individuals with head and neck cancer.Cancer Epidemiol. 2016 Jun;42:46-52. doi: 10.1016/j.canep.2016.03.003. Epub 2016 Mar 21. Cancer Epidemiol. 2016. PMID: 27010729 Free PMC article.
Cited by
-
Blood-based biomarkers of human papillomavirus-associated cancers: A systematic review and meta-analysis.Cancer. 2021 Mar 15;127(6):850-864. doi: 10.1002/cncr.33221. Epub 2020 Dec 3. Cancer. 2021. PMID: 33270909 Free PMC article.
-
Dietary behaviors and survival in people with head and neck cancer: Results from Head and Neck 5000.Head Neck. 2019 Jul;41(7):2074-2084. doi: 10.1002/hed.25660. Epub 2019 Jan 30. Head Neck. 2019. PMID: 30698303 Free PMC article.
-
HPV Detection in Head and Neck Squamous Cell Carcinomas: What Is the Issue?Front Oncol. 2020 Sep 15;10:1751. doi: 10.3389/fonc.2020.01751. eCollection 2020. Front Oncol. 2020. PMID: 33042820 Free PMC article. Review.
-
HPV - A different view on Head and Neck Cancer.Laryngorhinootologie. 2018 Mar;97(S 01):S48-S113. doi: 10.1055/s-0043-121596. Epub 2018 Mar 22. Laryngorhinootologie. 2018. PMID: 29905354 Free PMC article. Review.
-
Assessing the feasibility of a multimodal liquid biopsy for the diagnosis of HPV-associated oropharyngeal squamous cell carcinoma.Am J Clin Pathol. 2024 Jun 3;161(6):570-578. doi: 10.1093/ajcp/aqad185. Am J Clin Pathol. 2024. PMID: 38349613 Free PMC article.
References
-
- Hong AM, Grulich AE, Jones D, et al.Squamous cell carcinoma of the oropharynx in Australian males induced by human papillomavirus vaccine targets. Vaccine. 2010;2819:3269–3272. - PubMed
-
- Hammarstedt L, Lindquist D, Dahlstrand H, et al.Human papillomavirus as a risk factor for the increase in incidence of tonsillar cancer. Int J Cancer. 2006;11911:2620–2623. - PubMed
-
- Forte T, Niu J, Lockwood GA, et al.Incidence trends in head and neck cancers and human papillomavirus (HPV)-associated oropharyngeal cancer in Canada, 1992–2009. Cancer Causes Control. 2012;238:1343–1348. - PubMed
-
- Blomberg M, Nielsen A, Munk C, et al.Trends in head and neck cancer incidence in Denmark, 1978–2007: Focus on human papillomavirus associated sites. Int J Cancer. 2011;1293:733–741. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

