Molecular mechanisms underlying protective effects of quercetin against mitochondrial dysfunction and progressive dopaminergic neurodegeneration in cell culture and MitoPark transgenic mouse models of Parkinson's Disease
- PMID: 28376279
- PMCID: PMC5643047
- DOI: 10.1111/jnc.14033
Molecular mechanisms underlying protective effects of quercetin against mitochondrial dysfunction and progressive dopaminergic neurodegeneration in cell culture and MitoPark transgenic mouse models of Parkinson's Disease
Abstract
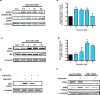
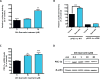
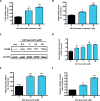
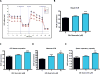
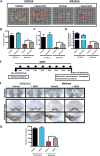
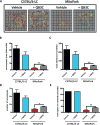
Quercetin, one of the major flavonoids in plants, has been recently reported to have neuroprotective effects against neurodegenerative processes. However, since the molecular signaling mechanisms governing these effects are not well clarified, we evaluated quercetin's effect on the neuroprotective signaling events in dopaminergic neuronal models and further tested its efficacy in the MitoPark transgenic mouse model of Parkinson's disease (PD). Western blot analysis revealed that quercetin significantly induced the activation of two major cell survival kinases, protein kinase D1 (PKD1) and Akt in MN9D dopaminergic neuronal cells. Furthermore, pharmacological inhibition or siRNA knockdown of PKD1 blocked the activation of Akt, suggesting that PKD1 acts as an upstream regulator of Akt in quercetin-mediated neuroprotective signaling. Quercetin also enhanced cAMP response-element binding protein phosphorylation and expression of the cAMP response-element binding protein target gene brain-derived neurotrophic factor. Results from qRT-PCR, Western blot analysis, mtDNA content analysis, and MitoTracker assay experiments revealed that quercetin augmented mitochondrial biogenesis. Quercetin also increased mitochondrial bioenergetics capacity and protected MN9D cells against 6-hydroxydopamine-induced neurotoxicity. To further evaluate the neuroprotective efficacy of quercetin against the mitochondrial dysfunction underlying PD, we used the progressive dopaminergic neurodegenerative MitoPark transgenic mouse model of PD. Oral administration of quercetin significantly reversed behavioral deficits, striatal dopamine depletion, and TH neuronal cell loss in MitoPark mice. Together, our findings demonstrate that quercetin activates the PKD1-Akt cell survival signaling axis and suggest that further exploration of quercetin as a promising neuroprotective agent for treating PD may offer clinical benefits.
Keywords: MitoPark; PGC-1α; PKD1; Parkinson's disease; mitochondrial biogenesis; quercetin.
© 2017 International Society for Neurochemistry.
Conflict of interest statement
A.G.K. and V.A. are shareholders of PK Biosciences Corporation (Ames, IA), which is interested in translating basic mechanistic studies into therapies. The other authors have no conflicts of interest.
Figures
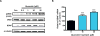


Similar articles
-
Mito-metformin protects against mitochondrial dysfunction and dopaminergic neuronal degeneration by activating upstream PKD1 signaling in cell culture and MitoPark animal models of Parkinson's disease.Front Neurosci. 2024 Feb 21;18:1356703. doi: 10.3389/fnins.2024.1356703. eCollection 2024. Front Neurosci. 2024. PMID: 38449738 Free PMC article.
-
Dopaminergic Neurons Exhibit an Age-Dependent Decline in Electrophysiological Parameters in the MitoPark Mouse Model of Parkinson's Disease.J Neurosci. 2016 Apr 6;36(14):4026-37. doi: 10.1523/JNEUROSCI.1395-15.2016. J Neurosci. 2016. PMID: 27053209 Free PMC article.
-
The MitoPark Mouse - an animal model of Parkinson's disease with impaired respiratory chain function in dopamine neurons.Parkinsonism Relat Disord. 2009 Dec;15 Suppl 3:S185-8. doi: 10.1016/S1353-8020(09)70811-9. Parkinsonism Relat Disord. 2009. PMID: 20082987 Review.
-
Manganese exposure exacerbates progressive motor deficits and neurodegeneration in the MitoPark mouse model of Parkinson's disease: Relevance to gene and environment interactions in metal neurotoxicity.Neurotoxicology. 2018 Jan;64:240-255. doi: 10.1016/j.neuro.2017.06.002. Epub 2017 Jun 20. Neurotoxicology. 2018. PMID: 28595911 Free PMC article.
-
Transgenic rodent models of Parkinson's disease.Acta Neurochir Suppl. 2008;101:89-92. doi: 10.1007/978-3-211-78205-7_15. Acta Neurochir Suppl. 2008. PMID: 18642640 Free PMC article. Review.
Cited by
-
Anti-Inflammatory Effects of Flavonoids in Common Neurological Disorders Associated with Aging.Int J Mol Sci. 2023 Feb 21;24(5):4297. doi: 10.3390/ijms24054297. Int J Mol Sci. 2023. PMID: 36901731 Free PMC article. Review.
-
Protective Effects of Flavonoids Against Mitochondriopathies and Associated Pathologies: Focus on the Predictive Approach and Personalized Prevention.Int J Mol Sci. 2021 Aug 11;22(16):8649. doi: 10.3390/ijms22168649. Int J Mol Sci. 2021. PMID: 34445360 Free PMC article. Review.
-
Ketone ester-enriched diet ameliorates motor and dopamine release deficits in MitoPark mice.Eur J Neurosci. 2024 Dec;60(11):6875-6890. doi: 10.1111/ejn.16601. Epub 2024 Nov 11. Eur J Neurosci. 2024. PMID: 39528410 Free PMC article.
-
Effectiveness of Flavonoid-Rich Diet in Alleviating Symptoms of Neurodegenerative Diseases.Foods. 2024 Jun 19;13(12):1931. doi: 10.3390/foods13121931. Foods. 2024. PMID: 38928874 Free PMC article. Review.
-
Mechanism of Yishen Chuchan decoction intervention of Parkinson's disease based on network pharmacology and experimental verification.Heliyon. 2024 Jul 18;10(14):e34823. doi: 10.1016/j.heliyon.2024.e34823. eCollection 2024 Jul 30. Heliyon. 2024. PMID: 39149067 Free PMC article.
References
-
- Anantharam V, Kitazawa M, Wagner J, Kaul S, Kanthasamy AG. Caspase-3-dependent proteolytic cleavage of protein kinase Cdelta is essential for oxidative stress-mediated dopaminergic cell death after exposure to methylcyclopentadienyl manganese tricarbonyl. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:1738–1751. - PMC - PubMed
-
- Anderson DW, Bradbury KA, Schneider JS. Neuroprotection in Parkinson models varies with toxin administration protocol. The European journal of neuroscience. 2006;24:3174–3182. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

