Enzymatically crosslinked silk-hyaluronic acid hydrogels
- PMID: 28376366
- PMCID: PMC5479139
- DOI: 10.1016/j.biomaterials.2017.03.046
Enzymatically crosslinked silk-hyaluronic acid hydrogels
Abstract
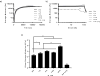
In this study, silk fibroin and hyaluronic acid (HA) were enzymatically crosslinked to form biocompatible composite hydrogels with tunable mechanical properties similar to that of native tissues. The formation of di-tyrosine crosslinks between silk fibroin proteins via horseradish peroxidase has resulted in a highly elastic hydrogel but exhibits time-dependent stiffening related to silk self-assembly and crystallization. Utilizing the same method of crosslinking, tyramine-substituted HA forms hydrophilic and bioactive hydrogels that tend to have limited mechanics and degrade rapidly. To address the limitations of these singular component scaffolds, HA was covalently crosslinked with silk, forming a composite hydrogel that exhibited both mechanical integrity and hydrophilicity. The composite hydrogels were assessed using unconfined compression and infrared spectroscopy to reveal of the physical properties over time in relation to polymer concentration. In addition, the hydrogels were characterized by enzymatic degradation and for cytotoxicity. Results showed that increasing HA concentration, decreased gelation time, increased degradation rate, and reduced changes that were observed over time in mechanics, water retention, and crystallization. These hydrogel composites provide a biologically relevant system with controllable temporal stiffening and elasticity, thus offering enhanced tunable scaffolds for short or long term applications in tissue engineering.
Keywords: Biomaterials; Enzymatic crosslinking; Hydrogel blends; Polymer composites; Temporal stiffening.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Figures




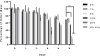

Similar articles
-
Molecular and macro-scale analysis of enzyme-crosslinked silk hydrogels for rational biomaterial design.Acta Biomater. 2017 Nov;63:76-84. doi: 10.1016/j.actbio.2017.09.020. Epub 2017 Sep 14. Acta Biomater. 2017. PMID: 28919509
-
Biomaterials from ultrasonication-induced silk fibroin-hyaluronic acid hydrogels.Biomacromolecules. 2010 Nov 8;11(11):3178-88. doi: 10.1021/bm1010504. Epub 2010 Oct 13. Biomacromolecules. 2010. PMID: 20942397
-
Ex vivo pregnant-like tissue model to assess injectable hydrogel for preterm birth prevention.J Biomed Mater Res B Appl Biomater. 2020 Feb;108(2):468-474. doi: 10.1002/jbm.b.34403. Epub 2019 May 9. J Biomed Mater Res B Appl Biomater. 2020. PMID: 31070848 Free PMC article.
-
Crosslinking strategies for silk fibroin hydrogels: promising biomedical materials.Biomed Mater. 2021 Feb 17;16(2):022004. doi: 10.1088/1748-605X/abb615. Biomed Mater. 2021. PMID: 33594992 Review.
-
Processing silk hydrogel and its applications in biomedical materials.Biotechnol Prog. 2015 May-Jun;31(3):630-40. doi: 10.1002/btpr.2058. Epub 2015 Mar 4. Biotechnol Prog. 2015. PMID: 25740113 Review.
Cited by
-
Accelerated Simple Preparation of Curcumin-Loaded Silk Fibroin/Hyaluronic Acid Hydrogels for Biomedical Applications.Polymers (Basel). 2023 Jan 18;15(3):504. doi: 10.3390/polym15030504. Polymers (Basel). 2023. PMID: 36771806 Free PMC article.
-
Antioxidative NAC-Loaded Silk Nanoparticles with Opening Mucosal Tight Junctions for Nasal Drug Delivery: An In Vitro and In Vivo Study.Pharmaceutics. 2022 Jun 17;14(6):1288. doi: 10.3390/pharmaceutics14061288. Pharmaceutics. 2022. PMID: 35745861 Free PMC article.
-
Biofabricating Functional Soft Matter Using Protein Engineering to Enable Enzymatic Assembly.Bioconjug Chem. 2018 Jun 20;29(6):1809-1822. doi: 10.1021/acs.bioconjchem.8b00197. Epub 2018 May 16. Bioconjug Chem. 2018. PMID: 29745651 Free PMC article. Review.
-
Statistical optimization of hydrazone-crosslinked hyaluronic acid hydrogels for protein delivery.J Mater Chem B. 2024 Mar 6;12(10):2523-2536. doi: 10.1039/d3tb01588b. J Mater Chem B. 2024. PMID: 38344905 Free PMC article.
-
Hyaluronic Acid in Biomedical Fields: New Trends from Chemistry to Biomaterial Applications.Int J Mol Sci. 2022 Nov 19;23(22):14372. doi: 10.3390/ijms232214372. Int J Mol Sci. 2022. PMID: 36430855 Free PMC article. Review.
References
-
- Caló E, Khutoryanskiy VV. Biomedical applications of hydrogels: a review of patents and commercial products. European Polymer Journal. 2015;65:252–267.
-
- Peppas NA, Hilt JZ, Khademhosseini A, Langer R. Hydrogels in biology and medicine: from molecular principles to bionanotechnology. Advanced Materials. 2006;18(11):1345–1360.
-
- Hoffman AS. Hydrogels for biomedical applications. Advanced drug delivery reviews. 2012;64:18–23. - PubMed
-
- Bae KH, Wang LS, Kurisawa M. Injectable biodegradable hydrogels: progress and challenges. Journal of Materials Chemistry B. 2013;1(40):5371–5388. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

