Adipogenic Differentiation of Mesenchymal Stem Cells Alters Their Immunomodulatory Properties in a Tissue-Specific Manner
- PMID: 28376564
- PMCID: PMC6052434
- DOI: 10.1002/stem.2622
Adipogenic Differentiation of Mesenchymal Stem Cells Alters Their Immunomodulatory Properties in a Tissue-Specific Manner
Abstract
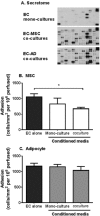
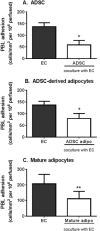
Chronic inflammation is associated with formation of ectopic fat deposits that might represent damage-induced aberrant mesenchymal stem cell (MSC) differentiation. Such deposits are associated with increased levels of inflammatory infiltrate and poor prognosis. Here we tested the hypothesis that differentiation from MSC to adipocytes in inflamed tissue might contribute to chronicity through loss of immunomodulatory function. We assessed the effects of adipogenic differentiation of MSC isolated from bone marrow or adipose tissue on their capacity to regulate neutrophil recruitment by endothelial cells and compared the differentiated cells to primary adipocytes from adipose tissue. Bone marrow derived MSC were immunosuppressive, inhibiting neutrophil recruitment to TNFα-treated endothelial cells (EC), but MSC-derived adipocytes were no longer able to suppress neutrophil adhesion. Changes in IL-6 and TGFβ1 signalling appeared critical for the loss of the immunosuppressive phenotype. In contrast, native stromal cells, adipocytes derived from them, and mature adipocytes from adipose tissue were all immunoprotective. Thus disruption of normal tissue stroma homeostasis, as occurs in chronic inflammatory diseases, might drive "abnormal" adipogenesis which adversely influences the behavior of MSC and contributes to pathogenic recruitment of leukocytes. Interestingly, stromal cells programmed in native fat tissue retain an immunoprotective phenotype. Stem Cells 2017;35:1636-1646.
Keywords: Adipocytes; Endothelial cells; Inflammation; Mesenchymal stem cells; Neutrophils; Tissue-specific.
© 2017 The Authors STEM CELLS published by Wiley Periodicals, Inc. on behalf of AlphaMed Press.
Figures


Similar articles
-
Crosstalk between mesenchymal stem cells and endothelial cells leads to downregulation of cytokine-induced leukocyte recruitment.Stem Cells. 2013 Dec;31(12):2690-702. doi: 10.1002/stem.1511. Stem Cells. 2013. PMID: 23939932
-
Adipose tissue-derived multipotent stromal cells have a higher immunomodulatory capacity than their bone marrow-derived counterparts.Stem Cells Transl Med. 2013 Jun;2(6):455-63. doi: 10.5966/sctm.2012-0184. Epub 2013 May 21. Stem Cells Transl Med. 2013. PMID: 23694810 Free PMC article.
-
Comparative Ability of Mesenchymal Stromal Cells from Different Tissues to Limit Neutrophil Recruitment to Inflamed Endothelium.PLoS One. 2016 May 12;11(5):e0155161. doi: 10.1371/journal.pone.0155161. eCollection 2016. PLoS One. 2016. PMID: 27171357 Free PMC article.
-
Thy-1: more than a marker for mesenchymal stromal cells.FASEB J. 2019 Jun;33(6):6689-6696. doi: 10.1096/fj.201802224R. Epub 2019 Feb 27. FASEB J. 2019. PMID: 30811954 Review.
-
Mesenchymal stem cell secretome and regenerative therapy after cancer.Biochimie. 2013 Dec;95(12):2235-45. doi: 10.1016/j.biochi.2013.05.010. Epub 2013 Jun 5. Biochimie. 2013. PMID: 23747841 Free PMC article. Review.
Cited by
-
Effect of hypoxia on proliferation and differentiation of induced pluripotent stem cell-derived mesenchymal stem cells.Heliyon. 2024 Oct 2;10(19):e38857. doi: 10.1016/j.heliyon.2024.e38857. eCollection 2024 Oct 15. Heliyon. 2024. PMID: 39421364 Free PMC article.
-
Controlled Release of Encapsuled Stromal-Derived Factor 1α Improves Bone Marrow Mesenchymal Stromal Cells Migration.Bioengineering (Basel). 2022 Dec 2;9(12):754. doi: 10.3390/bioengineering9120754. Bioengineering (Basel). 2022. PMID: 36550960 Free PMC article.
-
Mesenchymal Stem/Stromal Cells Derived from Human and Animal Perinatal Tissues-Origins, Characteristics, Signaling Pathways, and Clinical Trials.Cells. 2021 Nov 23;10(12):3278. doi: 10.3390/cells10123278. Cells. 2021. PMID: 34943786 Free PMC article. Review.
-
Effect of astragaloside IV on the immunoregulatory function of adipose-derived mesenchymal stem cells from patients with psoriasis vulgaris.J Tradit Chin Med. 2022 Aug;42(4):513-519. doi: 10.19852/j.cnki.jtcm.20220516.002. J Tradit Chin Med. 2022. PMID: 35848967 Free PMC article.
-
IFN-γ Licensing Does Not Enhance the Reduced Immunomodulatory Potential and Migratory Ability of Differentiation-Induced Porcine Bone Marrow-Derived Mesenchymal Stem Cells in an In Vitro Xenogeneic Application.Biomed Res Int. 2021 Sep 4;2021:4604856. doi: 10.1155/2021/4604856. eCollection 2021. Biomed Res Int. 2021. PMID: 34527737 Free PMC article.
References
-
- Munir H, McGettrick HM. Mesenchymal stem cells therapy for autoimmune disease: Risks and rewards. Stem Cells Dev 2015;24:2091–3100. - PubMed
-
- Le Blanc K, Mougiakakos D. Multipotent mesenchymal stromal cells and the innate immune system. Nat Rev Immunol 2012;12:383–396. - PubMed
-
- Luu N‐T, McGettrick HM, Buckley CD et al. Crosstalk between mesenchymal stem cells and endothelial cells leads to downregulation of cytokine‐induced leukocyte recruitment. Stem Cells 2013;31:2690–2702. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

