Impact of antiviral therapy on hepatocellular carcinoma and mortality in patients with chronic hepatitis C: systematic review and meta-analysis
- PMID: 28376711
- PMCID: PMC5379714
- DOI: 10.1186/s12876-017-0606-9
Impact of antiviral therapy on hepatocellular carcinoma and mortality in patients with chronic hepatitis C: systematic review and meta-analysis
Abstract
Background: The long-term clinical outcomes of antiviral therapy for patients with chronic hepatitis C are uncertain in terms of hepatitis C virus (HCV)-related morbidity and mortality according to the response to antiviral therapy. This study aimed to assess the impact of antiviral treatment on the development of HCC and mortality in patients with chronic HCV infection.
Methods: A systematic review was conducted for studies that evaluated the antiviral efficacy for patients with chronic hepatitis C or assessed the development of HCC or mortality between SVR (sustained virologic response) and non-SVR patients. The methodological quality of the enrolled publications was evaluated using Risk of Bias table or Newcastle-Ottawa scale. Random-effect model meta-analyses and meta-regression were performed. Publication bias was assessed.
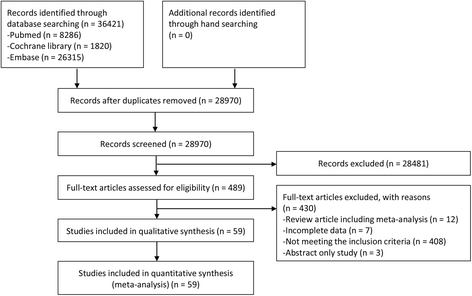
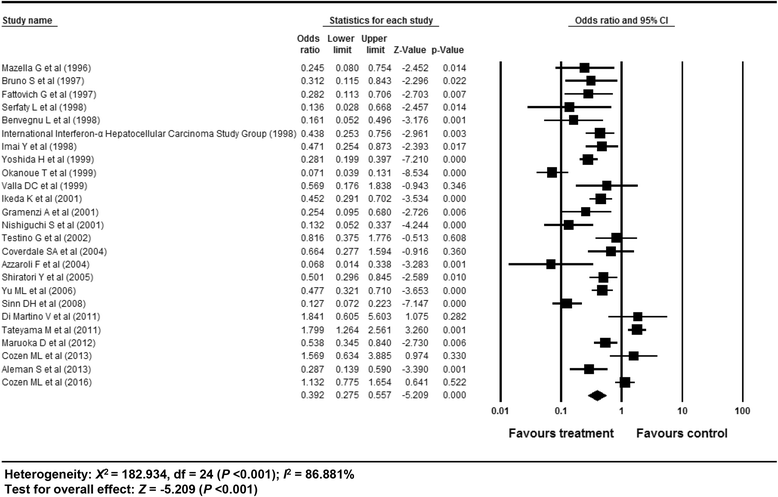
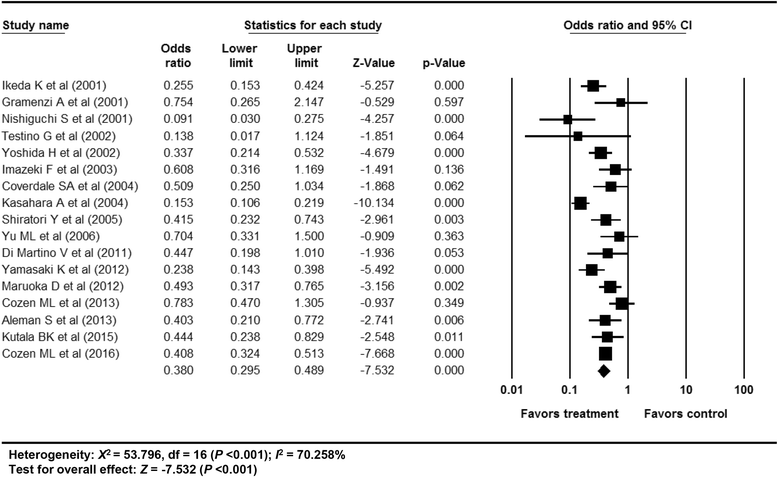
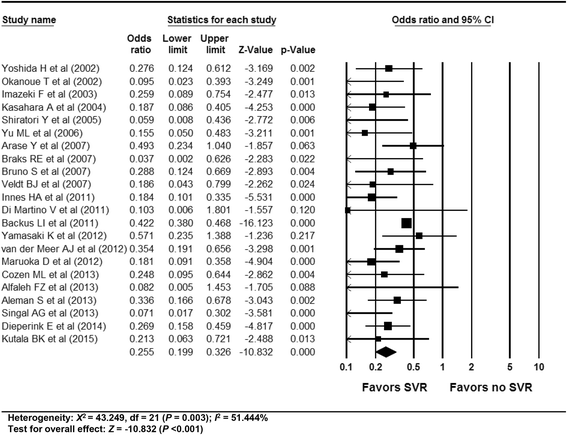
Results: In total, 59 studies (4 RCTs, 15 prospective and 40 retrospective cohort studies) were included. Antiviral treatment was associated with reduced development of HCC (vs. no treatment; OR 0.392, 95% CI 0.275-0.557), and this effect was intensified when SVR was achieved (vs. no SVR, OR: 0.203, 95% CI 0.164-0.251). Antiviral treatment was associated with lower all-cause mortality (vs. no treatment; OR 0.380, 95% CI 0.295-0.489) and liver-specific mortality (OR 0.363, 95% CI 0.260-0.508). This rate was also intensified when SVR was achieved [all-cause mortality (vs. no SVR, OR 0.255, 95% CI 0.199-0.326), liver-specific mortality (OR 0.126, 95% CI 0.094-0.169)]. Sensitivity analyses revealed robust results, and a small study effect was minimal.
Conclusions: In patients with chronic hepatitis C, antiviral therapy can reduce the development of HCC and mortality, especially when SVR is achieved.
Keywords: Antiviral therapy; Chronic hepatitis C; Hepatocellular carcinoma; Mortality; Sustained virologic response.
Figures
References
-
- Swain MG, Lai M, Shiffman ML, Cooksley WG, Zeuzem S, Dieterich DT, Abergel A, Pessôa MG, Lin A, Tietz A, et al. A sustained virologic response is durable in patients with chronic hepatitis C treated with peginterferon Alfa-2a and ribavirin. Gastroenterology. 2010;139:1593–601. doi: 10.1053/j.gastro.2010.07.009. - DOI - PubMed
-
- Martinot-Peignoux M, Stern C, Maylin S, Ripault MP, Boyer N, Leclere L, Castelnau C, Giuily N, El Ray A, Cardoso AC, et al. Twelve weeks posttreatment follow-up is as relevant as 24 weeks to determine the sustained virologic response in patients with hepatitis c virus receiving pegylated interferon and ribavirin. Hepatology. 2010;51:1122–6. doi: 10.1002/hep.23444. - DOI - PubMed
-
- Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions. Version 5.1.0. The Cochrane Collaboration; 2011, 2013.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

