Ethical issues in public health surveillance: a systematic qualitative review
- PMID: 28376752
- PMCID: PMC5381137
- DOI: 10.1186/s12889-017-4200-4
Ethical issues in public health surveillance: a systematic qualitative review
Abstract
Background: Public health surveillance is not ethically neutral and yet, ethics guidance and training for surveillance programmes is sparse. Development of ethics guidance should be based on comprehensive and transparently derived overviews of ethical issues and arguments. However, existing overviews on surveillance ethics are limited in scope and in how transparently they derived their results. Our objective was accordingly to provide an overview of ethical issues in public health surveillance; in addition, to list the arguments put forward with regards to arguably the most contested issue in surveillance, that is whether to obtain informed consent.
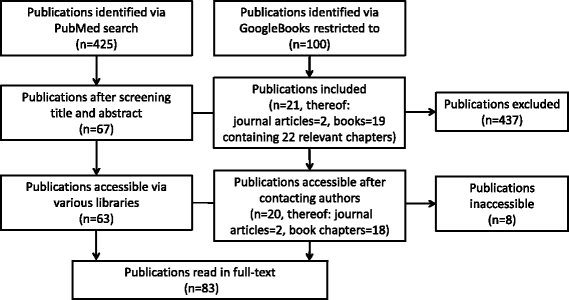
Methods: Ethical issues were defined based on principlism. We assumed an ethical issue to arise in surveillance when a relevant normative principle is not adequately considered or two principles come into conflict. We searched Pubmed and Google Books for relevant publications. We analysed and synthesized the data using qualitative content analysis.
Results: Our search strategy retrieved 525 references of which 83 were included in the analysis. We identified 86 distinct ethical issues arising in the different phases of the surveillance life-cycle. We further identified 20 distinct conditions that make it more or less justifiable to forego informed consent procedures.
Conclusions: This is the first systematic qualitative review of ethical issues in public health surveillance resulting in a comprehensive ethics matrix that can inform guidelines, reports, strategy papers, and educational material and raise awareness among practitioners.
Keywords: Ethics; Informed consent; Public health surveillance; Qualitative evidence synthesis; Systematic review.
Figures
References
-
- Fairchild AL, Bayer R, Colgrove J. Searching Eyes: Privacy, the State, and Disease Surveillance in America. Berkeley: University of California Press; 2007.
-
- International Health Regulation, 3rd edn. World Health Organization. 2016. http://www.who.int/ihr/publications/9789241580496/en/. Accessed 13 Oct 2016.
-
- Childress JF. Surveillance and Public Health Data: The Foundation and Eyes of Public Health. In: Bernheim RG, Childress JF, Bonnie RJ, Melnick AL, editors. Essentials of Public Health Ethics. Burlington: Jones & Bartlett Learning; 2015. pp. 97–118.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources