Heartmate 3 fully magnetically levitated left ventricular assist device for the treatment of advanced heart failure -1 year results from the Ce mark trial
- PMID: 28376837
- PMCID: PMC5379553
- DOI: 10.1186/s13019-017-0587-3
Heartmate 3 fully magnetically levitated left ventricular assist device for the treatment of advanced heart failure -1 year results from the Ce mark trial
Abstract
Background: The HeartMate 3 Left Ventricular Assist System (LVAS) (St. Jude Medical Inc., St Paul, MN) with full magnetic levitation allows for wide and consistent blood flow paths and an artificial pulse designed for enhanced hemocompatibility. The HeartMate 3 received market approval in the European Union in 2015 following completion of a multicenter study. After reaching the 6-month study endpoint, patients continue to be followed for 2 years with the 1-year results presented herein.
Methods: A prospective, non-randomized study included adults with advanced heart failure and ejection fraction (EF) ≤ 25%, cardiac index (CI) ≤ 2.2 L/min/m2 while not on inotropes, or inotrope dependent, or on optimal medical management for 45/60 days.
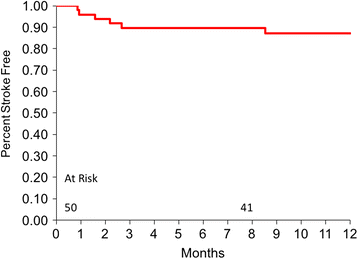
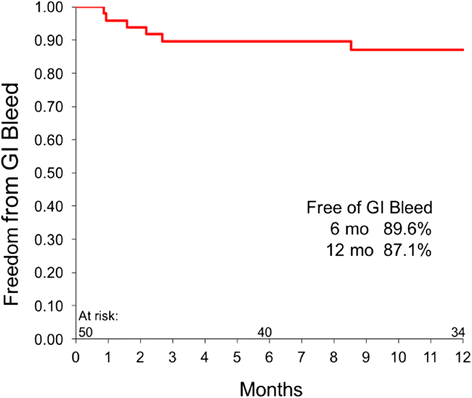
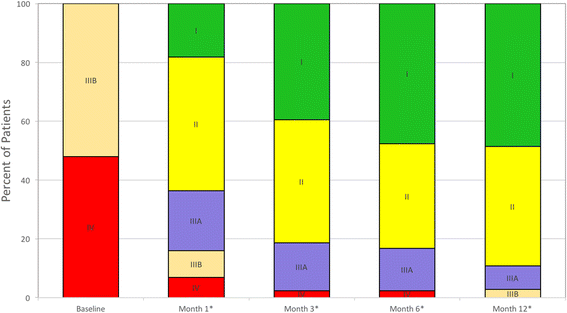
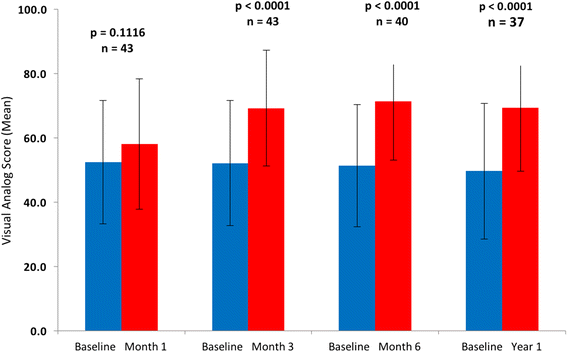
Results: Fifty patients-54% bridge to transplant (BTT) and 46% destination therapy (DT)-were enrolled and implanted with the HeartMate 3. At baseline, 92% of the patients were INTERMACS profiles 2-4, with cardiac index 1.8 + 0.5 L/min/m2 and 58% were supported with inotropes. At 1 year, 74% of the patients remain on support, 18% expired, 6% transplanted, and 2% explanted. The adverse events include 12% gastrointestinal bleeding, 16% driveline infections, 18% strokes, and 2% outflow graft thrombosis. There was no hemolysis, pump thrombosis or pump malfunction through 1 year. The six-minute walk test distance increased from a mean of 273 m to 371 m (P <0.0001). EQ-5D quality-of-life score increased from a mean of 52.7 to 70.8 (P = 0.0006).
Conclusions: The 1-year HeartMate 3 LVAS results show survival and adverse-event profile are similar to other approved devices, with no pump thrombosis or pump failure. Patient's functional status and quality of life significantly improved over time.
Trial registration: Clinicaltrials.gov registration number: NCT02170363 . Registered June 19, 2014.
Keywords: Heart failure; HeartMate 3; LVAS; Magnetic levitation.
Figures

References
-
- Starling RC, Naka Y, Boyle AJ, et al. Results of the post-U.S. Food and drug administration-approval study with a continuous flow left ventricular assist device as a bridge to heart transplantation a prospective study using the INTERMACS (interagency registry for mechanically assisted circulatory support) J Am Coll Cardiol. 2011;57:1890–1898. doi: 10.1016/j.jacc.2010.10.062. - DOI - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

