Systemic therapy in the curative treatment of head and neck squamous cell cancer: a systematic review
- PMID: 28376866
- PMCID: PMC5381126
- DOI: 10.1186/s40463-017-0199-x
Systemic therapy in the curative treatment of head and neck squamous cell cancer: a systematic review
Abstract
Objective: To review the available evidence and make recommendations regarding use of systemically administered drugs in combination or in sequence with radiation (RT) and/or surgery for cure and/or organ preservation in patients with locally advanced nonmetastatic (Stage III to IVB) squamous cell carcinoma of the head and neck (LASCCHN).
Method: Recognizing the Meta-analysis of Chemotherapy in Head and Neck Cancer (MACH-NC) group reports have de facto guided practice since 2000, we searched for systematic reviews in the MEDLINE, EMBASE and Cochrane Database of Systematic Reviews published from January 2000 to February 2015 in reference to 4 research questions. A search was also conducted for randomized trials (RCTs) up to February 2015 not included in the meta-analyses.
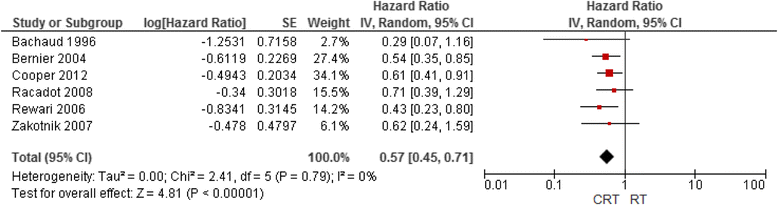
Result: The MACH-NC reports, 5 additional meta-analyses, and 30 RCTs not included by MACH-NC were identified. For chemotherapy, MACH-NC findings showing improved overall survival with concomitant chemoRT did not require modification. High-dose cisplatin was most commonly studied. We confirmed this benefit with cisplatin monotherapy in patients treated with with postoperative concurrent chemoRT. Other than cetuximab, no targeted agents and radiosensitizers studied in RCTs were shown effective. TPF induction chemotherapy was superior to PF for tumor response and larynx preservation but not survival. Larynx preservation was reported with both CRT and induction chemotherapy approaches.
Conclusion: ChemoRT with cisplatin at least 40 mg/m2 per week given as radical or postoperative adjuvant remains a standard treatment approach for LASCCHN that improves overall survival but increases toxicity. 5-FU plus platinum is supported by less data but may be a reasonable alternative for patients unsuitable for cisplatin. Of note, stratification of outcomes by HPV-status was not available but outcomes for oropharynx cancer appeared similar to other subsites in chemoRT RCTs. No RCTs have yet demonstrated superiority or non-inferiority of cetuximab-RT to CRT. In view of this, cetuximab-RT is suggested only for patients not candidates for CRT. Taxane-based triplet induction chemotherapy is superior to doublets for rapid tumour downsizing and for larynx preservation, but does not improve overall survival and should be used with primary G-CSF prophylaxis. Further investigation of induction approaches for larynx preservation may be warranted.
Keywords: Concurrent chemotherapy; Head and Neck; Human papilloma Virus; Induction chemotherapy; Locally advanced; Squamous cell carcinoma; Systematic review; Systemic chemotherapy.
Figures



References
-
- Schiff BA. Overview of Head and Neck Tumors - Tumors of the Head and Neck Merck Manual Professional Version. Retrieved from http://www.merckmanuals.com/en-ca/professional/ear,-nose,-andthroat-diso....
-
- Pignon JP, Bourhis J, Domenge C, Designe L. Chemotherapy added to locoregional treatment for head and neck squamous-cell carcinoma: three meta-analyses of updated individual data. MACH-NC Collaborative Group. Meta-Analysis of Chemotherapy on Head and Neck Cancer. Lancet. 2000;355(9208):949–55. doi: 10.1016/S0140-6736(00)90011-4. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

