Cancer immunotherapies targeting the PD-1 signaling pathway
- PMID: 28376884
- PMCID: PMC5381059
- DOI: 10.1186/s12929-017-0329-9
Cancer immunotherapies targeting the PD-1 signaling pathway
Abstract
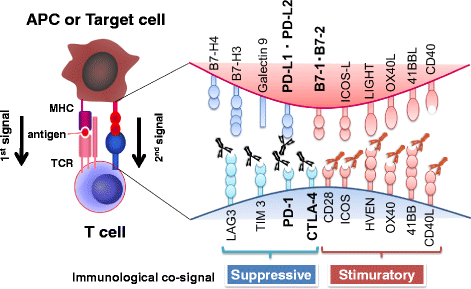
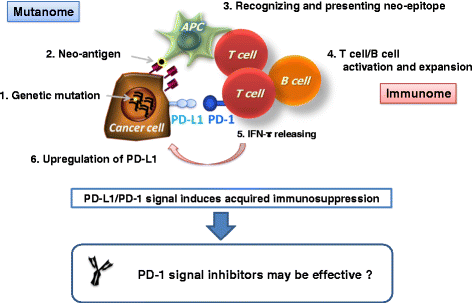
Immunotherapy has recently emerged as the fourth pillar of cancer treatment, joining surgery, radiation, and chemotherapy. While early immunotherapies focused on accelerating T-cell activity, current immune-checkpoint inhibitors take the brakes off the anti-tumor immune responses. Successful clinical trials with PD-1 monoclonal antibodies and other immune-checkpoint inhibitors have opened new avenues in cancer immunology. However, the failure of a large subset of cancer patients to respond to these new immunotherapies has led to intensified research on combination therapies and predictive biomarkers. Here we summarize the development of PD-1-blockade immunotherapy and current issues in its clinical use.
Keywords: Cancer immunotherapy; Immune checkpoint; PD-1; PD-L1.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

