Characterization of Brucella abortus mutant strain Δ22915, a potential vaccine candidate
- PMID: 28376905
- PMCID: PMC5381064
- DOI: 10.1186/s13567-017-0422-9
Characterization of Brucella abortus mutant strain Δ22915, a potential vaccine candidate
Abstract
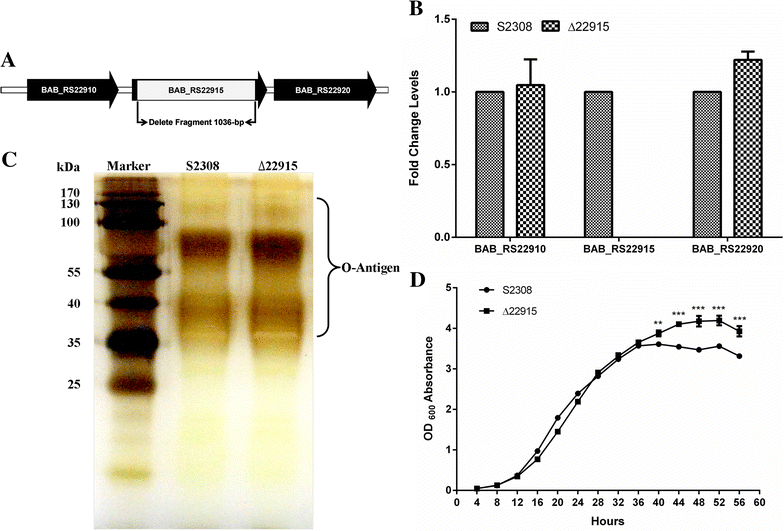
Brucellosis, caused by Brucella spp., is an important zoonosis worldwide. Vaccination is an effective strategy for protection against Brucella infection in livestock in developing countries and in wildlife in developed countries. However, current vaccine strains including S19 and RB51 are pathogenic to humans and pregnant animals, limiting their use. In this study, we constructed the Brucella abortus (B. abortus) S2308 mutant strain Δ22915, in which the putative lytic transglycosylase gene BAB_RS22915 was deleted. The biological properties of mutant strain Δ22915 were characterized and protection of mice against virulent S2308 challenge was evaluated. The mutant strain Δ22915 showed reduced survival within RAW264.7 cells and survival in vivo in mice. In addition, the mutant strain Δ22915 failed to escape fusion with lysosomes within host cells, and caused no observable pathological damage. RNA-seq analysis indicated that four genes associated with amino acid/nucleotide transport and metabolism were significantly upregulated in mutant strain Δ22915. Furthermore, inoculation of ∆22915 at 105 colony forming units induced effective host immune responses and long-term protection of BALB/c mice. Therefore, mutant strain ∆22915 could be used as a novel vaccine candidate in the future to protect animals against B. abortus infection.
Figures

References
-
- Pandey A, Cabello A, Akoolo L, Rice-Ficht A, Arenas-Gamboa A, McMurray D, Ficht TA, de Figueiredo P. The case for live attenuated vaccines against the neglected zoonotic diseases brucellosis and bovine tuberculosis. PLoS Negl Trop Dis. 2016;10:e0004572. doi: 10.1371/journal.pntd.0004572. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

