Head-to-head comparison of aggressive conventional therapy and three biological treatments and comparison of two de-escalation strategies in patients who respond to treatment: study protocol for a multicenter, randomized, open-label, blinded-assessor, phase 4 study
- PMID: 28376912
- PMCID: PMC5381054
- DOI: 10.1186/s13063-017-1891-x
Head-to-head comparison of aggressive conventional therapy and three biological treatments and comparison of two de-escalation strategies in patients who respond to treatment: study protocol for a multicenter, randomized, open-label, blinded-assessor, phase 4 study
Abstract
Background: New targeted therapies and improved treatment strategies have dramatically improved the outcomes of patients with rheumatoid arthritis (RA). However, it is unknown whether different early aggressive interventions can induce stable remission or a low-active disease state that can be maintained with conventional synthetic disease-modifying antirheumatic drug (csDMARD) therapy, and whether they differ in efficacy and safety. The Nordic Rheumatic Diseases Strategy Trials And Registries (NORD-STAR) study will assess and compare (1) the proportion of patients who achieve remission in a head-to-head comparison between csDMARD plus glucocorticoid therapy and three different biological DMARD (bDMARD) therapies with different modes of action and (2) two de-escalation strategies in patients who respond to first-line therapy.
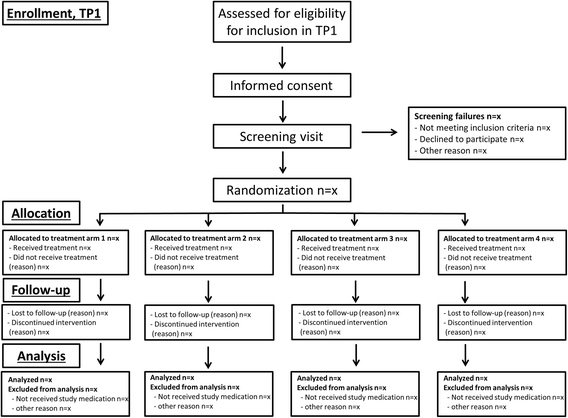
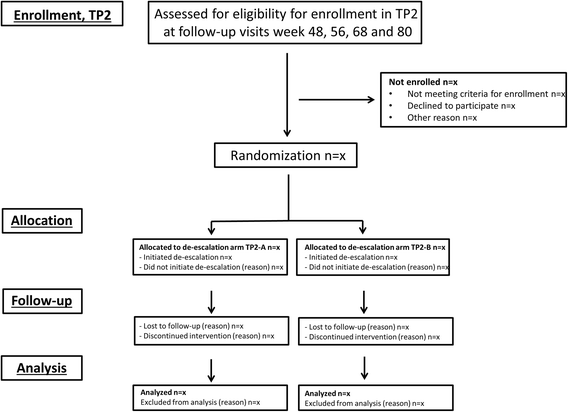
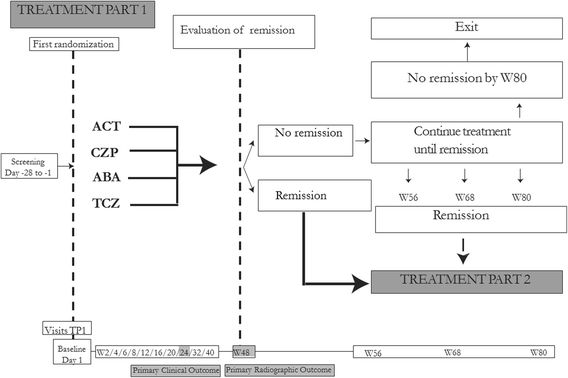
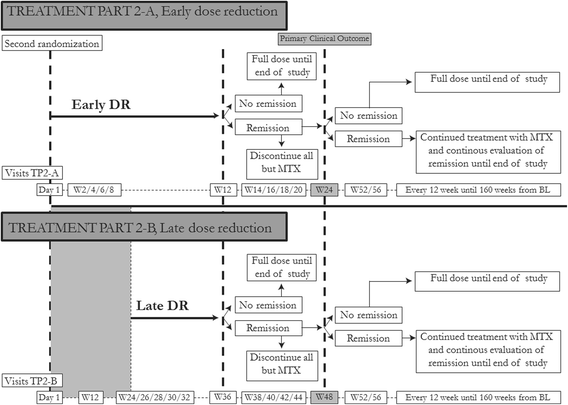
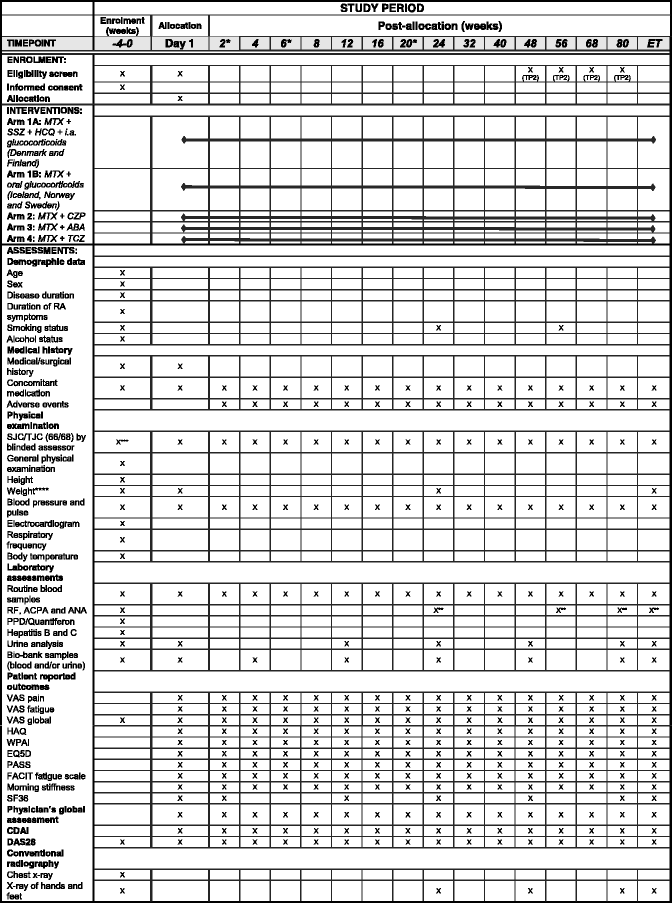
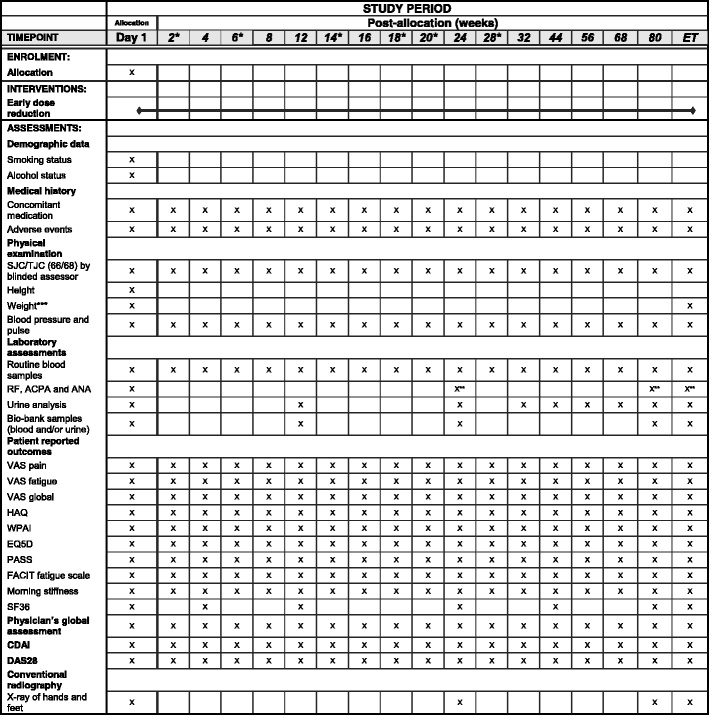
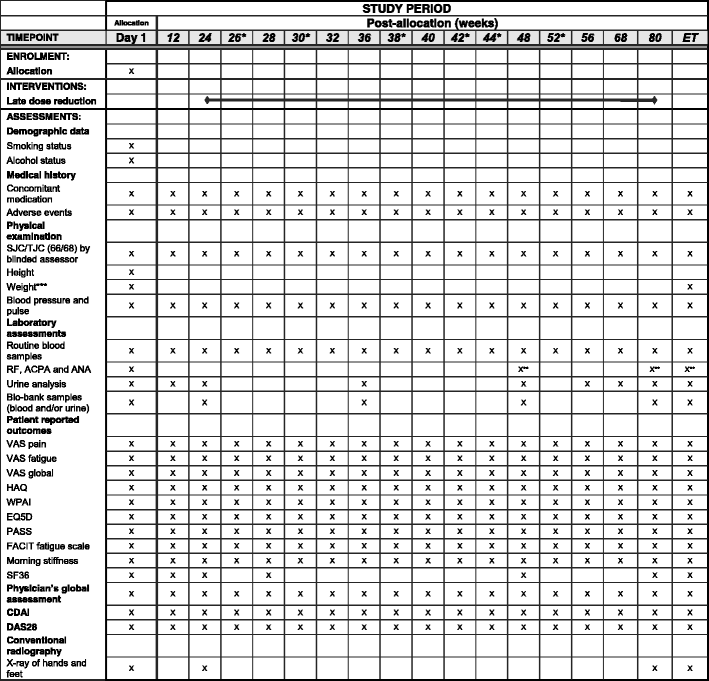
Methods/design: In a pragmatic, 80-160-week, multicenter, randomized, open-label, assessor-blinded, phase 4 study, 800 patients with early RA (symptom duration less than 24 months) are randomized 1:1:1:1 to one of four different treatment arms: (1) aggressive csDMARD therapy with methotrexate + sulphasalazine + hydroxychloroquine + i.a. glucocorticoids (arm 1A) or methotrexate + prednisolone p.o. (arm 1B), (2) methotrexate + certolizumab-pegol, (3) methotrexate + abatacept, or (4) methotrexate + tocilizumab. The primary clinical endpoint is the proportion of patients reaching Clinical Disease Activity Index (CDAI) remission at week 24. Patients in stable remission over 24 consecutive weeks enter part 2 of the study earliest after 48 weeks. Patients not achieving sustained CDAI remission over 24 consecutive weeks, exit the study after 80 weeks. In part 2, patients are re-randomized to two different de-escalation strategies, either immediate or delayed (after 24 weeks) tapering, followed by cessation of study medication. All patients remain on stable doses of methotrexate. The primary clinical endpoint in part 2 is the proportion of patients in remission (CDAI ≤2.8) 24 weeks after initiating treatment de-escalation. Radiographic assessment will be performed regularly throughout the trial, and blood and urine samples will be stored in a biobank for later biomarker analyses.
Discussion: NORD-STAR is the first investigator-initiated, randomized, early RA trial to compare (1) csDMARD and three different bDMARD therapies head to head and (2) two different de-escalation strategies. The trial has the potential to identify which treatment strategy to apply in early RA to achieve the best possible outcomes for both patients and society.
Trial registration: NCT01491815 and NCT02466581 . Registered on 8 December 2011 and May 2015, respectively. EudraCT: 2011-004720-35.
Keywords: Aggressive conventional treatment; Biological treatment; De-escalation strategy; Early treatment; Rheumatoid arthritis; Sustained remission.
Figures
Similar articles
-
Active conventional treatment and three different biological treatments in early rheumatoid arthritis: phase IV investigator initiated, randomised, observer blinded clinical trial.BMJ. 2020 Dec 2;371:m4328. doi: 10.1136/bmj.m4328. BMJ. 2020. PMID: 33268527 Free PMC article. Clinical Trial.
-
Effectiveness of TOcilizumab in comparison to Prednisone In Rheumatoid Arthritis patients with insufficient response to disease-modifying antirheumatic drugs (TOPIRA): study protocol for a pragmatic trial.Trials. 2020 Apr 5;21(1):313. doi: 10.1186/s13063-020-04260-y. Trials. 2020. PMID: 32248829 Free PMC article.
-
Certolizumab pegol, abatacept, tocilizumab or active conventional treatment in early rheumatoid arthritis: 48-week clinical and radiographic results of the investigator-initiated randomised controlled NORD-STAR trial.Ann Rheum Dis. 2023 Oct;82(10):1286-1295. doi: 10.1136/ard-2023-224116. Epub 2023 Jul 9. Ann Rheum Dis. 2023. PMID: 37423647 Clinical Trial.
-
Treating to target in established rheumatoid arthritis: Challenges and opportunities in an era of novel targeted therapies and biosimilars.Best Pract Res Clin Rheumatol. 2015 Aug-Dec;29(4-5):543-9. doi: 10.1016/j.berh.2015.10.001. Best Pract Res Clin Rheumatol. 2015. PMID: 26697765 Review.
-
Low rates of remission with methotrexate monotherapy in rheumatoid arthritis: review of randomised controlled trials could point towards a paradigm shift.RMD Open. 2019 Jul 27;5(2):e000993. doi: 10.1136/rmdopen-2019-000993. eCollection 2019. RMD Open. 2019. PMID: 31413870 Free PMC article. Review.
Cited by
-
Recent advances in the treatment of rheumatoid arthritis.Curr Opin Rheumatol. 2018 May;30(3):231-237. doi: 10.1097/BOR.0000000000000496. Curr Opin Rheumatol. 2018. PMID: 29461286 Free PMC article. Review.
-
Remission, response, retention and persistence to treatment with disease-modifying agents in patients with rheumatoid arthritis: a study of harmonised Swedish, Danish and Norwegian cohorts.RMD Open. 2023 Sep;9(3):e003027. doi: 10.1136/rmdopen-2023-003027. RMD Open. 2023. PMID: 37673441 Free PMC article.
-
Ultrasound assessment as predictor of disease relapse in children and adults with arthritis in clinical stable remission: new findings but still unmet needs.Ann Rheum Dis. 2018 Oct;77(10):1391-1393. doi: 10.1136/annrheumdis-2018-212941. Epub 2018 Jun 2. Ann Rheum Dis. 2018. PMID: 29860230 Free PMC article. No abstract available.
-
Glucocorticoid treatment in early rheumatoid arthritis is independently associated with increased PCSK9 levels: data from a randomised controlled trial.RMD Open. 2025 Jun 5;11(2):e005129. doi: 10.1136/rmdopen-2024-005129. RMD Open. 2025. PMID: 40480650 Free PMC article. Clinical Trial.
-
Transitional and CD21- PD-1+ B cells are associated with remission in early rheumatoid arthritis.BMC Rheumatol. 2025 Apr 21;9(1):45. doi: 10.1186/s41927-025-00487-x. BMC Rheumatol. 2025. PMID: 40259340 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

