Epidemiology of ACE Inhibitor Angioedema Utilizing a Large Electronic Health Record
- PMID: 28377081
- PMCID: PMC5476207
- DOI: 10.1016/j.jaip.2017.02.018
Epidemiology of ACE Inhibitor Angioedema Utilizing a Large Electronic Health Record
Abstract
Background: Angiotensin-converting enzyme inhibitors (ACEIs) are a common cause of drug-induced angioedema in the United States. Most epidemiologic ACEI angioedema data are from large multicenter clinical trials.
Objective: The objective of this study was to identify the incidence of and risk factors for ACEI angioedema using a large integrated electronic health record (EHR).
Methods: We conducted a retrospective cohort study of all ACEI prescriptions in the outpatient setting of a large academic center between January 1, 2000, and September 30, 2008. We determined frequency, timing, and risk factors for ACEI angioedema within 5 years of prescription. All data were derived from EHR sources, with angioedema defined by EHR reactions of angioedema, swelling, edema, or lip, eye, face, tongue, throat or mouth swelling.
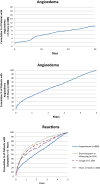
Results: Among 134,945 patients prescribed an ACEI, 0.7% (n = 888) developed angioedema during the subsequent 5 years. Sex was similar but patients who developed ACEI angioedema were younger (61.5 vs 62.7 years, P = .007). Patients with ACEI angioedema were more likely to have a history of nonsteroidal anti-inflammatory drug allergy compared with patients who did not develop angioedema (7.1% vs 4.2%, P < .001). We identified a 0.07% incidence of ACEI angioedema within 1 month of prescription and a 0.23% incidence during the first year. Incidence of angioedema was relatively constant annually over the subsequent 4 years (0.10% to 0.12%).
Conclusions: The incidence of ACEI angioedema within a large EHR is consistent with large clinical trial data. We observed a persistent and relatively constant annual risk; however, angioedema risk factors and underlying genetic and pathophysiological mechanisms require further study.
Keywords: Angioedema; Angiotensin-converting enzyme; Drug allergy; Drug hypersensitivity; Electronic health record; Epidemiology.
Copyright © 2017 American Academy of Allergy, Asthma & Immunology. Published by Elsevier Inc. All rights reserved.
Figures
References
-
- Ma J, Lee KV, Stafford RS. Changes in antihypertensive prescribing during US outpatient visits for uncomplicated hypertension between 1993 and 2004. Hypertension. 2006;48:846–852. - PubMed
-
- Messerli FH, Nussberger J. Vasopeptidase inhibition and angio-oedema. Lancet. 2000;356:608–609. - PubMed
-
- Kelly M, Donnelly JP, McAnnally JR, Wang HE. National estimates of emergency department visits for angioedema and allergic reactions in the United States. Allergy Asthma Proc. 2013;34:150–154. - PubMed
-
- Banerji A, Clark S, Blanda M, LoVecchio F, Snyder B, Camargo CA., Jr Multicenter study of patients with angiotensin-converting enzyme inhibitor-induced angioedema who present to the emergency department. Ann Allergy Asthma Immunol. 2008;100:327–332. - PubMed
-
- Gabb GM, Ryan P, Wing LM, Hutchinson KA. Epidemiological study of angioedema and ACE inhibitors. Aust N Z J Med. 1996;26:777–782. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous