Single low doses of MPTP decrease tyrosine hydroxylase expression in the absence of overt neuron loss
- PMID: 28377118
- PMCID: PMC5499677
- DOI: 10.1016/j.neuro.2017.03.008
Single low doses of MPTP decrease tyrosine hydroxylase expression in the absence of overt neuron loss
Abstract
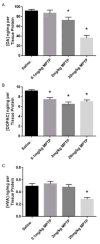
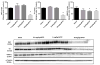
Parkinson's disease (PD) is the second most common age-related neurodegenerative disease. 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) is a prototypical neurotoxicant used in mice to mimic primary features of PD pathology including striatal dopamine depletion and dopamine neuron loss in the substantia nigra pars compacta (SNc). In the literature, there are several experimental paradigms involving multiple doses of MPTP that are used to elicit dopamine neuron loss. However, a recent study reported that a single low dose caused significant loss of dopamine neurons. Here, we determined the effect of a single intraperitoneal injection of one of three doses of MPTP (0.1, 2 and 20mg/kg) on dopamine neurons, labeled by tyrosine hydroxylase (TH+), and total neuron number (Nissl+) in the SNc using unbiased stereological counting. Data reveal a significant loss of neurons in the SNc (TH+ and Nissl+) only in the group treated with 20mg/kg MPTP. Groups treated with lower dose of MPTP (0.1 and 2mg/kg) only showed significant loss of TH+ neurons rather than TH+ and Nissl+ neurons. Striatal dopamine levels were decreased in the groups treated with 2 and 20mg/kg MPTP and striatal terminal markers including, TH and the dopamine transporter (DAT), were only decreased in the groups treated with 20mg/kg MPTP. These data demonstrate that lower doses of MPTP likely result in loss of TH expression rather than actual dopamine neuron loss in the SN. This finding reinforces the need to measure both total neuron number along with TH+ cells in determining dopamine neuron loss.
Keywords: Dopamine; Dopamine transporter; MPTP; Stereology; Tyrosine hydroxylase; Vesicular monoamine transporter.
Copyright © 2017 Elsevier B.V. All rights reserved.
Figures

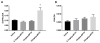
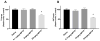
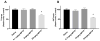
References
-
- Klingelhoefer L, Reichmann H. Pathogenesis of Parkinson disease[mdash]the gut-brain axis and environmental factors. Nat Rev Neurol. 2015;11(11):625–636. - PubMed
-
- Rodriguez-Oroz MC, et al. Initial clinical manifestations of Parkinson’s disease: features and pathophysiological mechanisms. The Lancet Neurology. 2009;8(12):1128–1139. - PubMed
-
- Masliah E, et al. Dopaminergic Loss and Inclusion Body Formation in α-Synuclein Mice: Implications for Neurodegenerative Disorders. Science. 2000;287(5456):1265–1269. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

