Cyp8b1 ablation prevents Western diet-induced weight gain and hepatic steatosis because of impaired fat absorption
- PMID: 28377401
- PMCID: PMC5582885
- DOI: 10.1152/ajpendo.00409.2016
Cyp8b1 ablation prevents Western diet-induced weight gain and hepatic steatosis because of impaired fat absorption
Abstract
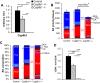
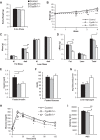
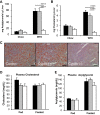
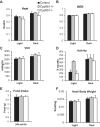
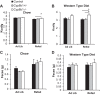
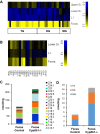
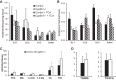
Bile acids (BAs) are cholesterol derivatives that regulate lipid metabolism, through their dual abilities to promote lipid absorption and activate BA receptors. However, different BA species have varying abilities to perform these functions. Eliminating 12α-hydroxy BAs in mice via Cyp8b1 knockout causes low body weight and improved glucose tolerance. The goal of this study was to determine mechanisms of low body weight in Cyp8b1-/- mice. We challenged Cyp8b1-/- mice with a Western-type diet and assessed body weight and composition. We measured energy expenditure, fecal calories, and lipid absorption and performed lipidomic studies on feces and intestine. We investigated the requirement for dietary fat in the phenotype using a fat-free diet. Cyp8b1-/- mice were resistant to Western diet-induced body weight gain, hepatic steatosis, and insulin resistance. These changes were associated with increased fecal calories, due to malabsorption of hydrolyzed dietary triglycerides. This was reversed by treating the mice with taurocholic acid, the major 12α-hydroxylated BA species. The improvements in body weight and steatosis were normalized by feeding mice a fat-free diet. The effects of BA composition on intestinal lipid handling are important for whole body energy homeostasis. Thus modulating BA composition is a potential tool for obesity or diabetes therapy.
Keywords: bile acids; diabetes; lipid absorption; obesity; triglyceride.
Copyright © 2017 the American Physiological Society.
Figures


References
-
- Ables GP, Yang KJ, Vogel S, Hernandez-Ono A, Yu S, Yuen JJ, Birtles S, Buckett LK, Turnbull AV, Goldberg IJ, Blaner WS, Huang LS, Ginsberg HN. Intestinal DGAT1 deficiency reduces postprandial triglyceride and retinyl ester excursions by inhibiting chylomicron secretion and delaying gastric emptying. J Lipid Res 53: 2364–2379, 2012. doi: 10.1194/jlr.M029041. - DOI - PMC - PubMed
-
- Akiyoshi T, Uchida K, Takase H, Nomura Y, Takeuchi N. Cholesterol gallstones in alloxan-diabetic mice. J Lipid Res 27: 915–924, 1986. - PubMed
-
- Borgstroem B. Influence of bile salt, pH, and time on the action of pancreatic lipase; physiological implications. J Lipid Res 5: 522–531, 1964. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

