Catabolism of the Last Two Steroid Rings in Mycobacterium tuberculosis and Other Bacteria
- PMID: 28377529
- PMCID: PMC5380842
- DOI: 10.1128/mBio.00321-17
Catabolism of the Last Two Steroid Rings in Mycobacterium tuberculosis and Other Bacteria
Abstract
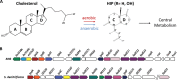
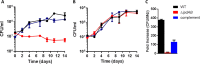
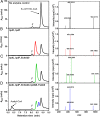
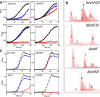
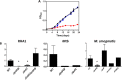
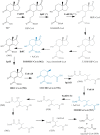
Most mycolic acid-containing actinobacteria and some proteobacteria use steroids as growth substrates, but the catabolism of the last two steroid rings has yet to be elucidated. In Mycobacterium tuberculosis, this pathway includes virulence determinants and has been proposed to be encoded by the KstR2-regulated genes, which include a predicted coenzyme A (CoA) transferase gene (ipdAB) and an acyl-CoA reductase gene (ipdC). In the presence of cholesterol, ΔipdC and ΔipdAB mutants of either M. tuberculosis or Rhodococcus jostii strain RHA1 accumulated previously undescribed metabolites: 3aα-H-4α(carboxyl-CoA)-5-hydroxy-7aβ-methylhexahydro-1-indanone (5-OH HIC-CoA) and (R)-2-(2-carboxyethyl)-3-methyl-6-oxocyclohex-1-ene-1-carboxyl-CoA (COCHEA-CoA), respectively. A ΔfadE32 mutant of Mycobacterium smegmatis accumulated 4-methyl-5-oxo-octanedioic acid (MOODA). Incubation of synthetic 5-OH HIC-CoA with purified IpdF, IpdC, and enoyl-CoA hydratase 20 (EchA20), a crotonase superfamily member, yielded COCHEA-CoA and, upon further incubation with IpdAB and a CoA thiolase, yielded MOODA-CoA. Based on these studies, we propose a pathway for the final steps of steroid catabolism in which the 5-member ring is hydrolyzed by EchA20, followed by hydrolysis of the 6-member ring by IpdAB. Metabolites accumulated by ΔipdF and ΔechA20 mutants support the model. The conservation of these genes in known steroid-degrading bacteria suggests that the pathway is shared. This pathway further predicts that cholesterol catabolism yields four propionyl-CoAs, four acetyl-CoAs, one pyruvate, and one succinyl-CoA. Finally, a ΔipdAB M. tuberculosis mutant did not survive in macrophages and displayed severely depleted CoASH levels that correlated with a cholesterol-dependent toxicity. Our results together with the developed tools provide a basis for further elucidating bacterial steroid catabolism and virulence determinants in M. tuberculosis.IMPORTANCE Bacteria are the only known steroid degraders, but the pathway responsible for degrading the last two steroid rings has yet to be elucidated. In Mycobacterium tuberculosis, this pathway includes virulence determinants. Using a series of mutants in M. tuberculosis and related bacteria, we identified a number of novel CoA thioesters as pathway intermediates. Analysis of the metabolites combined with enzymological studies establishes how the last two steroid rings are hydrolytically opened by enzymes encoded by the KstR2 regulon. Our results provide experimental evidence for novel ring-degrading enzymes, significantly advance our understanding of bacterial steroid catabolism, and identify a previously uncharacterized cholesterol-dependent toxicity that may facilitate the development of novel tuberculosis therapeutics.
Keywords: CoA thioester; Mycobacterium tuberculosis; catabolism; cholesterol; ring opening.
Copyright © 2017 Crowe et al.
Figures



References
-
- van der Geize R, Yam K, Heuser T, Wilbrink MH, Hara H, Anderton MC, Sim E, Dijkhuizen L, Davies JE, Mohn WW, Eltis LD. 2007. A gene cluster encoding cholesterol catabolism in a soil actinomycete provides insight into Mycobacterium tuberculosis survival in macrophages. Proc Natl Acad Sci U S A 104:1947–1952. doi: 10.1073/pnas.0605728104. - DOI - PMC - PubMed
-
- Yang FC, Chen YL, Tang SL, Yu CP, Wang PH, Ismail W, Wang CH, Ding JY, Yang CY, Yang CY, Chiang YR. 2016. Integrated multi-omics analyses reveal the biochemical mechanisms and phylogenetic relevance of anaerobic androgen biodegradation in the environment. ISME J 10:1967–1983. doi: 10.1038/ismej.2015.255. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
