ALS Pathogenesis and Therapeutic Approaches: The Role of Mesenchymal Stem Cells and Extracellular Vesicles
- PMID: 28377696
- PMCID: PMC5359305
- DOI: 10.3389/fncel.2017.00080
ALS Pathogenesis and Therapeutic Approaches: The Role of Mesenchymal Stem Cells and Extracellular Vesicles
Abstract
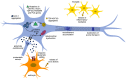
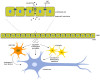
Amyotrophic lateral sclerosis (ALS) is a fatal neurodegenerative disease characterized by progressive muscle paralysis determined by the degeneration of motoneurons in the motor cortex brainstem and spinal cord. The ALS pathogenetic mechanisms are still unclear, despite the wealth of studies demonstrating the involvement of several altered signaling pathways, such as mitochondrial dysfunction, glutamate excitotoxicity, oxidative stress and neuroinflammation. To date, the proposed therapeutic strategies are targeted to one or a few of these alterations, resulting in only a minimal effect on disease course and survival of ALS patients. The involvement of different mechanisms in ALS pathogenesis underlines the need for a therapeutic approach targeted to multiple aspects. Mesenchymal stem cells (MSC) can support motoneurons and surrounding cells, reduce inflammation, stimulate tissue regeneration and release growth factors. On this basis, MSC have been proposed as promising candidates to treat ALS. However, due to the drawbacks of cell therapy, the possible therapeutic use of extracellular vesicles (EVs) released by stem cells is raising increasing interest. The present review summarizes the main pathological mechanisms involved in ALS and the related therapeutic approaches proposed to date, focusing on MSC therapy and their preclinical and clinical applications. Moreover, the nature and characteristics of EVs and their role in recapitulating the effect of stem cells are discussed, elucidating how and why these vesicles could provide novel opportunities for ALS treatment.
Keywords: ALS therapeutic applications; amyotrophic lateral sclerosis; exosomes; extracellular vesicles; mesenchymal stem cells.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

