Protective Effects of Dioscin against Lipopolysaccharide-Induced Acute Lung Injury through Inhibition of Oxidative Stress and Inflammation
- PMID: 28377715
- PMCID: PMC5359219
- DOI: 10.3389/fphar.2017.00120
Protective Effects of Dioscin against Lipopolysaccharide-Induced Acute Lung Injury through Inhibition of Oxidative Stress and Inflammation
Abstract
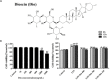
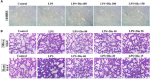
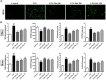
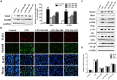
The protective effects of dioscin, a natural steroidal saponin from some medicinal plants including Dioscorea nipponica Makino, against lipopolysaccharide (LPS)- induced acute liver and renal damages have been reported in our previous works. However, the actions of dioscin against LPS-induced acute lung injury (ALI) is still unknown. In the present study, we investigated the effects and mechanisms of dioscin against LPS-induced ALI in vitro and in vivo. The results showed that dioscin obviously inhibited cell proliferation and markedly decreased reactive oxidative species level in 16HBE cells treated by LPS. In addition, dioscin significantly protected LPS-induced histological changes, inhibited the infiltration of inflammatory cells, as well as decreased the levels of MDA, SOD, NO and iNOS in mice and rats (p < 0.05). Mechanistically, dioscin significantly decreased the protein levels of TLR4, MyD88, TRAF6, TKB1, TRAF3, phosphorylation levels of PI3K, Akt, IκBα, NF-κB, and the mRNA levels of IL-1β, IL-6, and TNF-α against oxidative stress and inflammation (p < 0.05). Dioscin significantly reduced the overexpression of TLR4, and obviously down-regulated the levels of MyD88, TRAF6, TKB1, TRAF3, p-PI3K, p-Akt, p-IκBα, and p-NF-κB. These findings provide new perspectives for the study of ALI. Dioscin has protective effects on LPS-induced ALI via adjusting TLR4/MyD88- mediated oxidative stress and inflammation, which should be a potent drug in the treatment of ALI.
Keywords: TLR4 signal pathway; acute lung injury; dioscin; inflammation; lipopolysaccharide; oxidative stress.
Figures


References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

