Immunometabolic Regulation of Interleukin-17-Producing T Helper Cells: Uncoupling New Targets for Autoimmunity
- PMID: 28377767
- PMCID: PMC5359241
- DOI: 10.3389/fimmu.2017.00311
Immunometabolic Regulation of Interleukin-17-Producing T Helper Cells: Uncoupling New Targets for Autoimmunity
Abstract
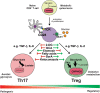
Interleukin-17-producing T helper (Th17) cells are critical for the host defense of bacterial and fungal pathogens and also play a major role in driving pathogenic autoimmune responses. Recent studies have indicated that the generation of Th17 cells from naïve CD4+ T cells is coupled with massive cellular metabolic adaptations, necessary to cope with different energy and metabolite requirements associated with switching from a resting to proliferative state. Furthermore, Th17 cells have to secure these metabolic adaptations when facing nutrient-limiting environments, such as at the sites of inflammation. Accumulating data indicates that this metabolic reprogramming is significantly linked to the differentiation of T helper cells and, particularly, that the metabolic changes of Th17 cells and anti-inflammatory Forkhead box P3+ regulatory T cells are tightly and reciprocally regulated. Thus, a better understanding of these processes could offer potential new targets for therapeutic interventions for autoimmune diseases. In this mini-review, we will highlight some of the recent advances and discoveries in the field, with a particular focus on metabolic demands of Th17 cells and their implications for autoimmunity.
Keywords: autoimmune diseases; glycolysis; immunometabolism; interleukin-17-producing T helper cells; oxidative phosphorylation; regulatory T cells.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

