Antibody-Dependent Cell-Mediated Cytotoxicity Epitopes on the Hemagglutinin Head Region of Pandemic H1N1 Influenza Virus Play Detrimental Roles in H1N1-Infected Mice
- PMID: 28377769
- PMCID: PMC5359280
- DOI: 10.3389/fimmu.2017.00317
Antibody-Dependent Cell-Mediated Cytotoxicity Epitopes on the Hemagglutinin Head Region of Pandemic H1N1 Influenza Virus Play Detrimental Roles in H1N1-Infected Mice
Abstract
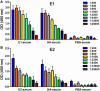
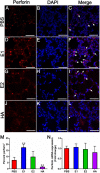
Engaging the antibody-dependent cell-mediated cytotoxicity (ADCC) for killing of virus-infected cells and secretion of antiviral cytokines and chemokines was incorporated as one of the important features in the design of universal influenza vaccines. However, investigation of the ADCC epitopes on the highly immunogenic influenza hemagglutinin (HA) head region has been rarely reported. In this study, we determined the ADCC and antiviral activities of two putative ADCC epitopes, designated E1 and E2, on the HA head of a pandemic H1N1 influenza virus in vitro and in a lethal mouse model. Our data demonstrated that sera from the E1-vaccinated mice could induce high ADCC activities. Importantly, the induction of ADCC response modestly decreased viral load in the lungs of H1N1-infected mice. However, the elevated ADCC significantly increased mouse alveolar damage and mortality than that of the PBS-vaccinated group (P < 0.0001). The phenotype was potentially due to an exaggerated inflammatory cell infiltration triggered by ADCC, as an upregulated release of cytotoxic granules (perforin) was observed in the lung tissue of E1-vaccinated mice after H1N1 influenza virus challenge. Overall, our data suggested that ADCC elicited by certain domains of HA head region might have a detrimental rather than protective effect during influenza virus infection. Thus, future design of universal influenza vaccine shall strike a balance between the induction of protective immunity and potential side effects of ADCC.
Keywords: H1N1 influenza virus; antibody-dependent cell-mediated cytotoxicity; hemagglutinin; lung damage; mice.
Figures

References
LinkOut - more resources
Full Text Sources
Other Literature Sources

