Astrocyte IKKβ/NF-κB signaling is required for diet-induced obesity and hypothalamic inflammation
- PMID: 28377875
- PMCID: PMC5369266
- DOI: 10.1016/j.molmet.2017.01.010
Astrocyte IKKβ/NF-κB signaling is required for diet-induced obesity and hypothalamic inflammation
Abstract
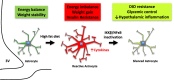
Objective: Obesity and high fat diet (HFD) consumption in rodents is associated with hypothalamic inflammation and reactive gliosis. While neuronal inflammation promotes HFD-induced metabolic dysfunction, the role of astrocyte activation in susceptibility to hypothalamic inflammation and diet-induced obesity (DIO) remains uncertain.
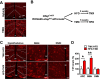
Methods: Metabolic phenotyping, immunohistochemical analyses, and biochemical analyses were performed on HFD-fed mice with a tamoxifen-inducible astrocyte-specific knockout of IKKβ (GfapCreERIkbkbfl/fl, IKKβ-AKO), an essential cofactor of NF-κB-mediated inflammation.
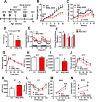
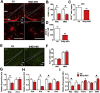
Results: IKKβ-AKO mice with tamoxifen-induced IKKβ deletion prior to HFD exposure showed equivalent HFD-induced weight gain and glucose intolerance as Ikbkbfl/fl littermate controls. In GfapCreERTdTomato marker mice treated using the same protocol, minimal Cre-mediated recombination was observed in the mediobasal hypothalamus (MBH). By contrast, mice pretreated with 6 weeks of HFD exposure prior to tamoxifen administration showed substantially increased recombination throughout the MBH. Remarkably, this treatment approach protected IKKβ-AKO mice from further weight gain through an immediate reduction of food intake and increase of energy expenditure. Astrocyte IKKβ deletion after HFD exposure-but not before-also reduced glucose intolerance and insulin resistance, likely as a consequence of lower adiposity. Finally, both hypothalamic inflammation and astrocytosis were reduced in HFD-fed IKKβ-AKO mice.
Conclusions: These data support a requirement for astrocytic inflammatory signaling in HFD-induced hyperphagia and DIO susceptibility that may provide a novel target for obesity therapeutics.
Keywords: ARC, arcuate nucleus; Agrp, Agouti-related peptide; Astrocytes; Bdnf, brain-derived neurotrophic factor; Cart, cocaine- and amphetamine-regulated transcript; Ccl2, C–C motif chemokine ligand 2; DIO, diet-induced obesity; DMH, dorsomedial hypothalamus; Energy homeostasis; GFAP, glial fibrillary acidic protein; GSIS, glucose-stimulated insulin secretion; GTT, glucose tolerance test; HFD, high-fat diet; Hypothalamus; IHC, immunohistochemistry; IKKβ, inhibitor of kappa B kinase beta; ITT, insulin tolerance test; Iba1, ionized calcium binding adaptor molecule 1; Il, interleukin; Inflammation; LPS, lipopolysaccharide; MBH, mediobasal hypothalamus; Metabolism; NF-κB, nuclear factor kappa B; Npy, neuropeptide Y; Obesity; Pomc, proopiomelanocortin; RER, respiratory exchange ratio; TMX, tamoxifen; Tnfa, tumor necrosis factor α; VMN, ventromedial nucleus; ir, immunoreactivity.
Figures

Similar articles
-
High fat induces acute and chronic inflammation in the hypothalamus: effect of high-fat diet, palmitate and TNF-α on appetite-regulating NPY neurons.Int J Obes (Lond). 2017 Jan;41(1):149-158. doi: 10.1038/ijo.2016.183. Epub 2016 Oct 24. Int J Obes (Lond). 2017. PMID: 27773938
-
Function of astrocyte MyD88 in high-fat-diet-induced hypothalamic inflammation.J Neuroinflammation. 2020 Jun 19;17(1):195. doi: 10.1186/s12974-020-01846-w. J Neuroinflammation. 2020. PMID: 32560726 Free PMC article.
-
Central inhibition of IKKβ/NF-κB signaling attenuates high-fat diet-induced obesity and glucose intolerance.Diabetes. 2015 Jun;64(6):2015-27. doi: 10.2337/db14-0093. Epub 2015 Jan 27. Diabetes. 2015. PMID: 25626735
-
Mechanistic insight into high-fat diet-induced metabolic inflammation in the arcuate nucleus of the hypothalamus.Biomed Pharmacother. 2021 Oct;142:112012. doi: 10.1016/j.biopha.2021.112012. Epub 2021 Aug 10. Biomed Pharmacother. 2021. PMID: 34388531 Review.
-
Dorsomedial hypothalamic NPY and energy balance control.Neuropeptides. 2012 Dec;46(6):309-14. doi: 10.1016/j.npep.2012.09.002. Epub 2012 Oct 18. Neuropeptides. 2012. PMID: 23083763 Free PMC article. Review.
Cited by
-
Obesity-induced inflammation: connecting the periphery to the brain.Nat Metab. 2024 Jul;6(7):1237-1252. doi: 10.1038/s42255-024-01079-8. Epub 2024 Jul 12. Nat Metab. 2024. PMID: 38997442 Review.
-
Elucidating the Interactive Roles of Glia in Alzheimer's Disease Using Established and Newly Developed Experimental Models.Front Neurol. 2018 Sep 26;9:797. doi: 10.3389/fneur.2018.00797. eCollection 2018. Front Neurol. 2018. PMID: 30319529 Free PMC article. Review.
-
Obesity-associated microglial inflammatory activation paradoxically improves glucose tolerance.Cell Metab. 2023 Sep 5;35(9):1613-1629.e8. doi: 10.1016/j.cmet.2023.07.008. Epub 2023 Aug 11. Cell Metab. 2023. PMID: 37572666 Free PMC article.
-
Cellular Contributors to Hypothalamic Inflammation in Obesity.Mol Cells. 2020 May 31;43(5):431-437. doi: 10.14348/molcells.2020.0055. Mol Cells. 2020. PMID: 32392909 Free PMC article. Review.
-
Investigating Gene-Gene and Gene-Environment Interactions in the Association Between Overnutrition and Obesity-Related Phenotypes.Front Genet. 2019 Mar 4;10:151. doi: 10.3389/fgene.2019.00151. eCollection 2019. Front Genet. 2019. PMID: 30886629 Free PMC article.
References
-
- Hotamisligil G.S. Inflammation and metabolic disorders. Nature. 2006;444(7121):860–867. - PubMed
-
- De Souza C.T., Araujo E.P., Bordin S., Ashimine R., Zollner R.L., Boschero A.C. Consumption of a fat-rich diet activates a proinflammatory response and induces insulin resistance in the hypothalamus. Endocrinology. 2005;146(10):4192–4199. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

