Host Response to Staphylococcus epidermidis Colonization and Infections
- PMID: 28377905
- PMCID: PMC5359315
- DOI: 10.3389/fcimb.2017.00090
Host Response to Staphylococcus epidermidis Colonization and Infections
Abstract
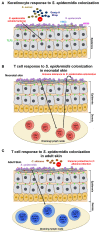
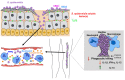
The majority of research in the Staphylococcus field has been dedicated to the understanding of Staphylococcus aureus infections. In contrast, there is limited information on infections by coagulase-negative Staphylococci (CoNS) and how the host responds to them. S. epidermidis, a member of the coagulase-negative Staphylococci, is an important commensal organism of the human skin and mucous membranes; and there is emerging evidence of its benefit for human health in fighting off harmful microorganisms. However, S. epidermidis can cause opportunistic infections, which include particularly biofilm-associated infections on indwelling medical devices. These often can disseminate into the bloodstream; and in fact, S. epidermidis is the most frequent cause of nosocomial sepsis. The increasing use of medical implants and the dramatic shift in the patient demographic population in recent years have contributed significantly to the rise of S. epidermidis infections. Furthermore, treatment has been complicated by the emergence of antibiotic-resistant strains. Today, S. epidermidis is a major nosocomial pathogen posing significant medical and economic burdens. In this review, we present the current understanding of mechanisms of host defense against the prototypical CoNS species S. epidermidis as a commensal of the skin and mucous membranes, and during biofilm-associated infection and sepsis.
Keywords: Staphylococcus epidermidis; biofilm-associated infection; biofilms; coagulase-negative staphylococci; host defense; innate immunity; sepsis.
Figures
References
-
- Bloom B., Schelonka R., Kueser T., Walker W., Jung E., Kaufman D., et al. (2005). Multicenter study to assess safety and efficacy of INH-A21, a donor-selected human staphylococcal immunoglobulin, for prevention of nosocomial infections in very low birth weight infants. Pediatr. Infect. Dis. J. 24, 858–866. 10.1097/01.inf.0000180504.66437.1f - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

