Mesenchymal Stem Cell-Derived Exosomes: Immunomodulatory Evaluation in an Antigen-Induced Synovitis Porcine Model
- PMID: 28377922
- PMCID: PMC5359696
- DOI: 10.3389/fvets.2017.00039
Mesenchymal Stem Cell-Derived Exosomes: Immunomodulatory Evaluation in an Antigen-Induced Synovitis Porcine Model
Abstract
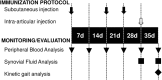
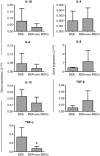
Synovitis is an inflammatory process associated with pain, disability, and discomfort, which is usually treated with anti-inflammatory drugs or biological agents. Mesenchymal stem cells (MSCs) have been also successfully used in the treatment of inflammatory-related diseases such as synovitis or arthritis. In the last years, the exosomes derived from MSCs have become a promising tool for the treatment of inflammatory-related diseases and their therapeutic effect is thought to be mediated (at least in part) by their immunomodulatory potential. In this work, we aimed to evaluate the anti-inflammatory effect of these exosomes in an antigen-induced synovitis animal model. To our knowledge, this is the first report where exosomes derived from MSCs have been evaluated in an animal model of synovitis. Our results demonstrated a decrease of synovial lymphocytes together with a downregulation of TNF-α transcripts in those exosome-treated joints. These results support the immunomodulatory effect of these exosomes and point out that they may represent a promising therapeutic option for the treatment of synovitis.
Keywords: exosomes; immunomodulation; inflammation; mesenchymal stem cells; synovitis.
Figures


References
LinkOut - more resources
Full Text Sources
Other Literature Sources

