Primary human osteoblasts with reduced alkaline phosphatase and matrix mineralization baseline capacity are responsive to extremely low frequency pulsed electromagnetic field exposure - Clinical implication possible
- PMID: 28377966
- PMCID: PMC5365212
- DOI: 10.1016/j.bonr.2015.08.002
Primary human osteoblasts with reduced alkaline phosphatase and matrix mineralization baseline capacity are responsive to extremely low frequency pulsed electromagnetic field exposure - Clinical implication possible
Abstract
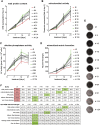
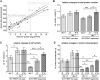
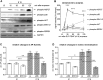
For many years electromagnetic fields (EMFs) have been used clinically with various settings as an exogenous stimulation method to promote fracture healing. However, underlying mechanisms of action and EMF parameters responsible for certain effects remain unclear. Our aim was to investigate the influence of defined EMFs on human osteoblasts' and osteoclasts' viability and function. Primary human osteoblasts and osteoclasts were treated 3 times weekly for 21 days during their maturation process using the Somagen® device (Sachtleben GmbH, Hamburg, Germany), generating defined extremely low-frequency pulsed electromagnetic fields (ELF-PEMFs). Certain ELF-PEMF treatment significantly increased the total protein content (up to 66%), mitochondrial activity (up to 91.1%) and alkaline phosphatase (AP) activity (up to 129.9%) of human osteoblasts during the entire differentiation process. Furthermore, ELF-PEMF treatment enhanced formation of mineralized matrix (up to 276%). Interestingly, ELF-PEMF dependent induction of AP activity and matrix mineralization was strongly donor dependent - only osteoblasts with a poor initial osteoblast function responded to the ELF-PEMF treatment. As a possible regulatory mechanism, activation of the ERK1/2 signaling pathway was identified. Maturation of osteoclasts from human monocytes was not affected by the ELF-PEMF treatment. In summary the results indicate that a specific ELF-PEMF treatment with the Somagen® device improves viability and maturation of osteoblasts, while osteoclast viability and maturation was not affected. Hence, ELF-PEMF might represent an interesting adjunct to conventional therapy supporting bone formation during fracture healing or even for the treatment of osteoporosis.
Keywords: ERK1/2; Extremely low-frequency pulsed electromagnetic fields (ELF-PEMF); Human osteoblasts; Human osteoclasts; Specific EMF-responsiveness.
Figures



References
-
- Aaron R.K., Ciombor D.M., Simon B.J. Treatment of nonunions with electric and electromagnetic fields. Clin. Orthop. Relat. Res. 2004;21–9 - PubMed
-
- Alexander D., Schafer F., Olbrich M., Friedrich B., Buhring H.J., Hoffmann J., Reinert S. MSCA-1/TNAP selection of human jaw periosteal cells improves their mineralization capacity. Cell. Physiol. Biochem. 2010;26:1073–1080. - PubMed
-
- Bassett C.A., Pawluk R.J., Pilla A.A. Acceleration of fracture repair by electromagnetic fields. A surgically noninvasive method. Ann. N. Y. Acad. Sci. 1974;238:242–262. - PubMed
-
- Bassett C.A., Pawluk R.J., Pilla A.A. Augmentation of bone repair by inductively coupled electromagnetic fields. Science. 1974;184:575–577. - PubMed
-
- Bawin S.M., Kaczmarek L.K., Adey W.R. Effects of modulated VHF fields on the central nervous system. Ann. N. Y. Acad. Sci. 1975;247:74–81. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

