Adherence and Persistence Across Antidepressant Therapeutic Classes: A Retrospective Claims Analysis Among Insured US Patients with Major Depressive Disorder (MDD)
- PMID: 28378157
- PMCID: PMC5425490
- DOI: 10.1007/s40263-017-0417-0
Adherence and Persistence Across Antidepressant Therapeutic Classes: A Retrospective Claims Analysis Among Insured US Patients with Major Depressive Disorder (MDD)
Erratum in
-
Erratum to: Adherence and Persistence Across Antidepressant Therapeutic Classes: A Retrospective Claims Analysis Among Insured US Patients with Major Depressive Disorder (MDD).CNS Drugs. 2017 Jun;31(6):511. doi: 10.1007/s40263-017-0435-y. CNS Drugs. 2017. PMID: 28451963 Free PMC article. No abstract available.
Abstract
Background: Adherence and persistence to therapy, or how well a patient follows provider directions on frequency and time to discontinuation of prescribed medications, is associated with positive health outcomes, including decreased healthcare costs and patient mortality. A clear literature gap exists assessing adherence and persistence to antidepressants (ADs) in the major depressive disorder (MDD) population at clinically relevant time points and at the therapeutic class level.
Objective: This study assessed adherence and persistence to specific ADs, therapeutic classes, and AD therapy overall at multiple time points among US individuals from commercial, Medicare supplemental, and Medicaid insurance plans.
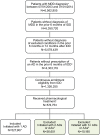
Methods: Patients with MDD without AD or MDD claims in the prior 6 months who initiated therapy in 2003-2014 with a selective serotonin reuptake inhibitor (SSRI), serotonin and norepinephrine reuptake inhibitor (SNRI), tricyclic AD (TCA), monoamine oxidase inhibitor (MAOI), or other AD were identified using MarketScan® databases. These databases contain information on diagnoses, billing codes, and dates of service. Adherence (proportion of days covered) and persistence (days until a 30-day gap in therapy) were calculated to AD medication, AD therapeutic class, and AD therapy overall over the first 3, 6, 9, and 12 months from the index prescription date. Multivariable logistic regression estimated the adjusted odds ratios (ORs) of adherence to initial AD medication comparing AD therapeutic classes.
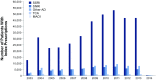
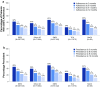
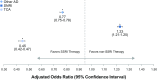
Results: For 527,907 patients, adherence to initial AD medication decreased over 3, 6, 9, and 12 months (41, 31, 24, and 21%, respectively). Similar patterns were observed for adherence to initial AD therapeutic class, AD therapy overall, and all three persistence calculations. The odds of adherence to SNRIs versus SSRIs were 20-27% greater at 3, 6, 9, and 12 months (ORs 1.20, 1.23, 1.25, 1.27, respectively; p-values all <0.0001). Similar or significantly lower odds of adherence were demonstrated for other classes versus SSRIs at 3, 6, 9, and 12 months [ORs for other ADs 0.80, 0.77, 0.74, 0.72, respectively (p-values all <0.0001); ORs for TCAs 0.46, 0.45, 0.47, 0.49, respectively (p-values all <0.0001); ORs for MAOIs 1.13, 1.0, 0.77, 0.69, respectively (p-values all >0.05)].
Conclusion: We found low adherence and persistence to ADs in the MDD population. Within the limitations of the insurance claims data we analysed, our results suggest that adherence may differ based on therapeutic class, as patients initiating SNRI therapy appeared to have a higher likelihood of adherence versus SSRIs over the year assessed, while the odds of adherence appeared similar or lower for other classes versus SSRIs. Further prospective research is needed to confirm these findings and determine additional drivers of these apparent differences by AD therapeutic class.
Conflict of interest statement
Funding
This research was funded as part of the University of Washington/Allergan Fellowship Program through the University of Washington's Pharmaceutical Outcomes Research and Policy Program, as well as by Allergan. Open access fees were funded by Allergan.
Conflicts of interest
Katelyn R. Keyloun and Patrick Gillard are full-time employees of Allergan. Zsolt Hepp was an employee of Allergan at the time of the study. Michael E. Thase has received grants from the Agency for Healthcare Research and Quality, Alkermes, AssureRx, Avanir, Forest Laboratories (an Allergan affiliate), Intracellular, Janssen, the National Institute of Mental Health, Otsuka, PharmaNeuroboost, Roche, and Takeda; has acted as an advisor or consultant for Acadia, Alkermes, Allergan (Forest Laboratories, Naurex), AstraZeneca, Bristol-Myers Squibb, Cerecor, Eli Lilly, Fabre-Kramer Pharmaceuticals, Inc., Gerson Lehman Group, GlaxoSmithKline, Guidepoint Global, Johnson and Johnson (Janssen, Ortho-McNeil), Lundbeck, MedAvante, Merck, Moksha8, Nestle (PamLab), Neuronetics, Novartis, Otsuka, Pfizer, Shire, Sunovion, and Takeda; has received royalties from the American Psychiatric Association, Guilford Publications, Herald House, and W. W. Norton & Company; and holds equity in MedAvante Inc. Ryan N. Hansen and Emily Beth Devine have no conflicts to report.
Figures



Comment in
-
Psychopharmacology in the Age of "Big Data": The Promises and Limitations of Electronic Prescription Records.CNS Drugs. 2017 May;31(5):417-419. doi: 10.1007/s40263-017-0419-y. CNS Drugs. 2017. PMID: 28378158 Free PMC article. No abstract available.
References
-
- Andrade L, Caraveo-Anduaga JJ, Berglund P, Bijl RV, De Graaf R, Vollebergh W, et al. The epidemiology of major depressive episodes: results from the International Consortium of Psychiatric Epidemiology (ICPE) Surveys. Int J Methods Psychiatr Res. 2003;12(1):3–21. doi: 10.1002/mpr.138. - DOI - PMC - PubMed
-
- Substance Abuse and Mental Health Services Administration. Results from the 2013 National Survey on Drug Use and Health: Mental Health Findings. 2014. http://www.samhsa.gov/data/sites/default/files/NSDUHmhfr2013/NSDUHmhfr20.... Accessed 7 July 2016. - PubMed
-
- American Psychiatric Association (APA). Practice guidelines for the treatment of patients with major depressive disorder, 3rd edition. Arlington, VA: APA; 2010. http://psychiatryonline.org/guidelines. Accessed 7 July 2016.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

