IL-5-stimulated eosinophils adherent to periostin undergo stereotypic morphological changes and ADAM8-dependent migration
- PMID: 28378503
- PMCID: PMC5623171
- DOI: 10.1111/cea.12934
IL-5-stimulated eosinophils adherent to periostin undergo stereotypic morphological changes and ADAM8-dependent migration
Abstract
Background: IL-5 causes suspended eosinophils to polarize with filamentous (F)-actin and granules at one pole and the nucleus in a specialized uropod, the "nucleopod," which is capped with P-selectin glycoprotein ligand-1 (PSGL-1). IL-5 enhances eosinophil adhesion and migration on periostin, an extracellular matrix protein upregulated in asthma by type 2 immunity mediators.
Objective: Determine how the polarized morphology evolves to foster migration of IL-5-stimulated eosinophils on a surface coated with periostin.
Methods: Blood eosinophils adhering to adsorbed periostin were imaged at different time points by fluorescent microscopy, and migration of eosinophils on periostin was assayed.
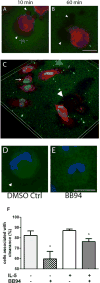
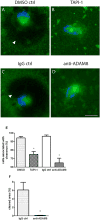
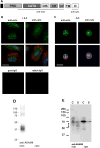
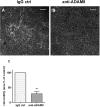
Results: After 10 minutes in the presence of IL-5, adherent eosinophils were polarized with PSGL-1 at the nucleopod tip and F-actin distributed diffusely at the opposite end. After 30-60 minutes, the nucleopod had dissipated such that PSGL-1 was localized in a crescent or ring away from the cell periphery, and F-actin was found in podosome-like structures. The periostin layer, detected with monoclonal antibody Stiny-1, shown here to recognize the FAS1 4 module, was cleared in wide areas around adherent eosinophils. Clearance was attenuated by metalloproteinase inhibitors or antibodies to disintegrin metalloproteinase 8 (ADAM8), a major eosinophil metalloproteinase previously implicated in asthma pathogenesis. ADAM8 was not found in podosome-like structures, which are associated with proteolytic activity in other cell types. Instead, immunoblotting demonstrated proteoforms of ADAM8 that lack the cytoplasmic tail in the supernatant. Anti-ADAM8 inhibited migration of IL-5-stimulated eosinophils on periostin.
Conclusions and clinical relevance: Migrating IL-5-activated eosinophils on periostin exhibit loss of nucleopodal features and appearance of prominent podosomes along with clearance of the Stiny-1 periostin epitope. Migration and epitope clearance are both attenuated by inhibitors of ADAM8. We propose, therefore, that eosinophils remodel and migrate on periostin-rich extracellular matrix in the asthmatic airway in an ADAM8-dependent manner, making ADAM8 a possible therapeutic target.
Keywords: ADAM8; FAS1 modules; IL-5; Stiny-1; eosinophils; migration; periostin; podosomes.
© 2017 John Wiley & Sons Ltd.
Conflict of interest statement
MWJ received a fee for consulting from Guidepoint Global, a fee from Genentech for speaking, and funds for research from Hoffmann-La Roche; and is an advisory board member for Genentech. The other authors have no conflicts of interest.
Figures

Similar articles
-
α(M)β(2) integrin-mediated adhesion and motility of IL-5-stimulated eosinophils on periostin.Am J Respir Cell Mol Biol. 2013 Apr;48(4):503-10. doi: 10.1165/rcmb.2012-0150OC. Am J Respir Cell Mol Biol. 2013. PMID: 23306834 Free PMC article. Clinical Trial.
-
Control of cytokine-driven eosinophil migratory behavior by TGF-beta-induced protein (TGFBI) and periostin.PLoS One. 2018 Jul 26;13(7):e0201320. doi: 10.1371/journal.pone.0201320. eCollection 2018. PLoS One. 2018. PMID: 30048528 Free PMC article.
-
IL-5 induces suspended eosinophils to undergo unique global reorganization associated with priming.Am J Respir Cell Mol Biol. 2014 Mar;50(3):654-64. doi: 10.1165/rcmb.2013-0181OC. Am J Respir Cell Mol Biol. 2014. PMID: 24156300 Free PMC article.
-
ADAM8 in allergy.Inflamm Allergy Drug Targets. 2008 Jun;7(2):108-12. doi: 10.2174/187152808785107598. Inflamm Allergy Drug Targets. 2008. PMID: 18691140 Review.
-
ADAM8 in invasive cancers: links to tumor progression, metastasis, and chemoresistance.Clin Sci (Lond). 2019 Jan 11;133(1):83-99. doi: 10.1042/CS20180906. Print 2019 Jan 15. Clin Sci (Lond). 2019. PMID: 30635388 Review.
Cited by
-
The Multiple Roles of Periostin in Non-Neoplastic Disease.Cells. 2022 Dec 22;12(1):50. doi: 10.3390/cells12010050. Cells. 2022. PMID: 36611844 Free PMC article. Review.
-
Comorbidity of Neurally Mediated Syncope and Allergic Disease in Children.Front Immunol. 2020 Aug 28;11:1865. doi: 10.3389/fimmu.2020.01865. eCollection 2020. Front Immunol. 2020. PMID: 32983103 Free PMC article. Review.
-
Role of periostin in ECRS.Eur Arch Otorhinolaryngol. 2021 Aug;278(8):2665-2672. doi: 10.1007/s00405-020-06369-x. Epub 2020 Sep 21. Eur Arch Otorhinolaryngol. 2021. PMID: 32954441 Free PMC article. Review.
-
The Airway Epithelium-A Central Player in Asthma Pathogenesis.Int J Mol Sci. 2020 Nov 24;21(23):8907. doi: 10.3390/ijms21238907. Int J Mol Sci. 2020. PMID: 33255348 Free PMC article. Review.
-
Eosinophil cytolysis on Immunoglobulin G is associated with microtubule formation and suppression of rho-associated protein kinase signalling.Clin Exp Allergy. 2020 Feb;50(2):198-212. doi: 10.1111/cea.13538. Epub 2019 Dec 9. Clin Exp Allergy. 2020. PMID: 31750580 Free PMC article.
References
-
- Thomas A, Busse WW. The evolving role of eosinophils in asthma. In: Lee JJ, Rosenberg HF eds. Eosinophils in health and disease. Amsterdam: Elsevier. 2013:448–62.
-
- Arron JR, Townsend MJ, Keir ME, Yaspan BL, Chan AC. Stratified medicine in inflammatory disorders: From theory to practice. Clin Immunol. 2015;161:11–22. - PubMed
-
- Kay AB, Phipps S, Robinson DS. A role for eosinophils in airway remodelling in asthma. Trends Immunol. 2004;25:477–82. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

