Opsonophagocytic Antibodies to Serotype Ia, Ib, and III Group B Streptococcus among Korean Infants and in Intravenous Immunoglobulin Products
- PMID: 28378545
- PMCID: PMC5383604
- DOI: 10.3346/jkms.2017.32.5.737
Opsonophagocytic Antibodies to Serotype Ia, Ib, and III Group B Streptococcus among Korean Infants and in Intravenous Immunoglobulin Products
Abstract
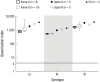
Group B streptococcus (GBS) infection is a leading cause of sepsis and meningitis among infants, and is associated with high rates of morbidity and mortality in many countries. Protection against GBS typically involves antibody-mediated opsonization by phagocytes and complement components. The present study evaluated serotype-specific functional antibodies to GBS among Korean infants and in intravenous immunoglobulin (IVIG) products. An opsonophagocytic killing assay (OPA) was used to calculate the opsonization indices (OIs) of functional antibodies to serotypes Ia, Ib, and III in 19 IVIG products from 5 international manufacturers and among 98 Korean infants (age: 0-11 months). The GBS Ia, Ib, and III serotypes were selected because they are included in a trivalent GBS vaccine formulation that is being developed. The OI values for the IVIG products were 635-5,706 (serotype Ia), 488-1,421 (serotype Ib), and 962-3,315 (serotype III), and none of the IVIG lots exhibited undetectable OI values (< 4). The geometric mean OI values were similar for all 3 serotypes when we compared the Korean manufacturers. The seropositive rate among infants was significantly lower for serotype Ia (18.4%), compared to serotype Ib and serotype III (both, 38.8%). Infant age of ≥ 3 months was positively correlated with the seropositive rates for each serotype. Therefore, only a limited proportion of infants exhibited protective immunity against serotype Ia, Ib, and III GBS infections. IVIG products that exhibit high antibody titers may be a useful therapeutic or preventive measure for infants. Further studies are needed to evaluate additional serotypes and age groups.
Keywords: Antibodies; Immunoglobulins; Infant; Intravenous; Opsonin Proteins; Streptococcus agalactiae.
© 2017 The Korean Academy of Medical Sciences.
Conflict of interest statement
The authors have no potential conflicts of interest to disclose.
Figures


References
-
- Phares CR, Lynfield R, Farley MM, Mohle-Boetani J, Harrison LH, Petit S, Craig AS, Schaffner W, Zansky SM, Gershman K, et al. Epidemiology of invasive group B streptococcal disease in the United States, 1999-2005. JAMA. 2008;299:2056–2065. - PubMed
-
- Le Doare K, Heath PT. An overview of global GBS epidemiology. Vaccine. 2013;31(Suppl 4):D7–12. - PubMed
-
- Baker CJ, Kasper DL. Correlation of maternal antibody deficiency with susceptibility to neonatal group B streptococcal infection. N Engl J Med. 1976;294:753–756. - PubMed
-
- Boyer KM, Papierniak CK, Gadzala CA, Parvin JD, Gotoff SP. Transplacental passage of IgG antibody to group B streptococcus serotype Ia. J Pediatr. 1984;104:618–620. - PubMed
-
- Gotoff SP, Papierniak CK, Klegerman ME, Boyer KM. Quantitation of IgG antibody to the type-specific polysaccharide of group B streptococcus type 1b in pregnant women and infected infants. J Pediatr. 1984;105:628–630. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

