A Single Center Analysis of the Positivity of Hepatitis B Antibody after Neonatal Vaccination Program in Korea
- PMID: 28378555
- PMCID: PMC5383614
- DOI: 10.3346/jkms.2017.32.5.810
A Single Center Analysis of the Positivity of Hepatitis B Antibody after Neonatal Vaccination Program in Korea
Abstract
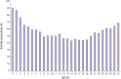
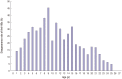
The antibody to hepatitis B surface antigen (anti-HBs) seropositivity rate after 3 doses of hepatitis B virus (HBV) vaccination during infancy period is known to be higher than 90%. However, a considerable number of vaccines do not form protective anti-HBs or chronologic decrease of anti-HBs. We retrospectively collected data of HBV serologic test results in 20,738 individuals from 2000 to 2015. After exclusion criteria were applied, 19,072 individuals were included. We analyzed the anti-HBs seropositivity rate, anti-HBs disappearance rate, anti-HBs positive seroconversion rate after receiving a booster vaccine, and the difference in anti-HBs positivity between the 2 groups; group A (born before 2005, while both recombinant vaccines and plasma-derived vaccines were used) and group B (born after 2005, when only recombinant vaccines were used by national regulation). The anti-HBs seropositivity rate was 55.8%, but there was a significant difference in the rate of seropositivity for anti-HBs between the group A and B (53.0% vs. 78.1%, P < 0.001). There was no significant age-adjusted difference in the mean seropositivity rate between the 2 groups (P = 0.058). In addition, the anti-HBs positivity rate was significantly lower in the group A as compared with the group B during infancy (83.1% vs. 92.1%, P < 0.001). A total of 1,106 anti-HBs-positive subjects underwent serologic tests more than twice. Of these, 217 subjects (19.6%) showed anti-HBs disappearance. After booster vaccinations, 87.4% (83/95) achieved seroconversion from seronegative to seropositive. Our results highlight the importance of lifelong protection against HBV and the possible necessity of booster vaccination after adolescent period.
Keywords: Booster; Children; Hepatitis B antibody; Plasma-derived Vaccine; Recombinant DNA Vaccine; South Korea.
© 2017 The Korean Academy of Medical Sciences.
Conflict of interest statement
The authors have no potential conflicts of interest to disclose.
Figures
References
-
- Chun BY, Lee MK, Rho YK. The prevalence of hepatitis B surface antigen among Korean by literature review. Korean J Epidemiol. 1992;14:70–78.
-
- Choe YH, Seo JK, Yun JH, Lee HS. Recent changes in prevalence of hepatitis B viral markers in preschool children in Seoul, 1995. J Korean Pediatr Soc. 1996;39:1254–1259.
-
- Jang MK, Lee JY, Lee JH, Kim YB, Kim HY, Yoo JY. The investigation for the change of HBsAg positive rate of grade junior high high-schoolers for recent 3 years in Kangwon province. Korean J Med. 2000;58:608–615.
-
- Choe BH. The epidemiology and present status of chronic hepatitis B in Korean children. Korean J Pediatr. 2008;51:696–703.
-
- Roberts H, Kruszon-Moran D, Ly KN, Hughes E, Iqbal K, Jiles RB, Holmberg SD. Prevalence of chronic hepatitis B virus (HBV) infection in U.S. households: National Health and Nutrition Examination Survey (NHANES), 1988-2012. Hepatology. 2016;63:388–397. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

