Siglec-7 restores β-cell function and survival and reduces inflammation in pancreatic islets from patients with diabetes
- PMID: 28378743
- PMCID: PMC5381285
- DOI: 10.1038/srep45319
Siglec-7 restores β-cell function and survival and reduces inflammation in pancreatic islets from patients with diabetes
Abstract
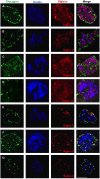
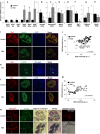
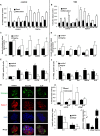
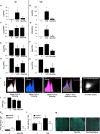
Chronic inflammation plays a key role in both type 1 and type 2 diabetes. Cytokine and chemokine production within the islets in a diabetic milieu results in β-cell failure and diabetes progression. Identification of targets, which both prevent macrophage activation and infiltration into islets and restore β-cell functionality is essential for effective diabetes therapy. We report that certain Sialic-acid-binding immunoglobulin-like-lectins (siglecs) are expressed in human pancreatic islets in a cell-type specific manner. Siglec-7 was expressed on β-cells and down-regulated in type 1 and type 2 diabetes and in infiltrating activated immune cells. Over-expression of Siglec-7 in diabetic islets reduced cytokines, prevented β-cell dysfunction and apoptosis and reduced recruiting of migrating monocytes. Our data suggest that restoration of human Siglec-7 expression may be a novel therapeutic strategy targeted to both inhibition of immune activation and preservation of β-cell function and survival.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


References
-
- Donath M. Y., Storling J., Maedler K. & Mandrup-Poulsen T. Inflammatory mediators and islet beta-cell failure: a link between type 1 and type 2 diabetes. J. Mol. Med. 81, 455–470 (2003). - PubMed
-
- Tisch R. & McDevitt H. Insulin-dependent diabetes mellitus. Cell 85, 291–297 (1996). - PubMed
-
- Mokdad A. H. et al.. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA 289, 76–79 (2003). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

