Study of the tensile properties of individual multicellular fibres generated by Bacillus subtilis
- PMID: 28378797
- PMCID: PMC5380956
- DOI: 10.1038/srep46052
Study of the tensile properties of individual multicellular fibres generated by Bacillus subtilis
Abstract
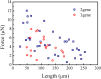
Multicellular fibres formed by Bacillus subtilis (B. subtilis) are attracting interest because of their potential application as degradable biomaterials. However, mechanical properties of individual fibres remain unknown because of their small dimensions. Herein, a new approach is developed to investigate the tensile properties of individual fibres with an average diameter of 0.7 μm and a length range of 25.7-254.3 μm. Variations in the tensile strengths of fibres are found to be the result of variable interactions among pairs of microbial cells known as septa. Using Weibull weakest-link model to study this mechanical variability, we predict the length effect of the sample. Moreover, the mechanical properties of fibres are found to depend highly on relative humidity (RH), with a brittle-ductile transition occurring around RH = 45%. The elastic modulus is 5.8 GPa in the brittle state, while decreases to 62.2 MPa in the ductile state. The properties of fibres are investigated by using a spring model (RH < 45%) for its elastic behaviour, and the Kelvin-Voigt model (RH > 45%) for the time-dependent response. Loading-unloading experiments and numerical calculations demonstrate that necking instability comes from structural changes (septa) and viscoelasticity dominates the deformation of fibres at high RH.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



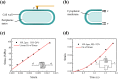

 are 2.1 and 63.3 MPa, respectively; for multicellular fibres with two genes deleted, they are 1.8 and 82.5 MPa, respectively. (c) Tensile strength at high RH. The parameters m and
are 2.1 and 63.3 MPa, respectively; for multicellular fibres with two genes deleted, they are 1.8 and 82.5 MPa, respectively. (c) Tensile strength at high RH. The parameters m and  are 1.7 and 33.7 MPa, respectively, for fibres with three genes deleted.
are 1.7 and 33.7 MPa, respectively, for fibres with three genes deleted.



Similar articles
-
Mechanical properties of Bacillus subtilis cell walls: effects of removing residual culture medium.J Bacteriol. 1991 Jan;173(1):197-203. doi: 10.1128/jb.173.1.197-203.1991. J Bacteriol. 1991. PMID: 1898920 Free PMC article.
-
Mechanical properties of peptidoglycan as determined from bacterial thread.Int J Biol Macromol. 1989 Aug;11(4):201-6. doi: 10.1016/0141-8130(89)90069-x. Int J Biol Macromol. 1989. PMID: 2518734
-
Mechanical properties of Bacillus subtilis cell walls: effects of ions and lysozyme.J Bacteriol. 1991 Jan;173(1):204-10. doi: 10.1128/jb.173.1.204-210.1991. J Bacteriol. 1991. PMID: 1898921 Free PMC article.
-
Mechanical properties of bioactive glass 9-93 fibres.Acta Biomater. 2006 Jan;2(1):103-7. doi: 10.1016/j.actbio.2005.08.008. Epub 2005 Oct 6. Acta Biomater. 2006. PMID: 16701864
-
Mechanical behaviour of degradable phosphate glass fibres and composites-a review.Biomed Mater. 2015 Dec 23;11(1):014105. doi: 10.1088/1748-6041/11/1/014105. Biomed Mater. 2015. PMID: 26694533 Review.
Cited by
-
Biophysical characterization of synthetic adhesins for predicting and tuning engineered living material properties.Matter. 2024 Jun 5;7(6):2125-2143. doi: 10.1016/j.matt.2024.03.019. Epub 2024 Apr 22. Matter. 2024. PMID: 39165662 Free PMC article.
-
Mechanics of Bacterial Interaction and Death on Nanopatterned Surfaces.Biophys J. 2021 Jan 19;120(2):217-231. doi: 10.1016/j.bpj.2020.12.003. Epub 2020 Dec 15. Biophys J. 2021. PMID: 33333030 Free PMC article.
-
A Multiscale Material Testing System for In Situ Optical and Electron Microscopes and Its Application.Sensors (Basel). 2017 Aug 4;17(8):1800. doi: 10.3390/s17081800. Sensors (Basel). 2017. PMID: 28777341 Free PMC article.
References
-
- Kumar V., Bhardwaj Y. K., Rawat K. P. & Sabharwal S. Radiation-induced grafting of vinylbenzyltrimethylammonium chloride (VBT) onto cotton fabric and study of its anti-bacterial activities. Radiat. Phys. Chem. 73, 175–182 (2005).
-
- Hakimi O., Knight D. P., Vollrath F. & Vadgama P. Spider and mulberry silkworm silks as compatible biomaterials. Compos. Pt. B: Eng. 38, 324–337 (2007).
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

