Alpha-lipoic acid ameliorates the epithelial mesenchymal transition induced by unilateral ureteral obstruction in mice
- PMID: 28378840
- PMCID: PMC5380949
- DOI: 10.1038/srep46065
Alpha-lipoic acid ameliorates the epithelial mesenchymal transition induced by unilateral ureteral obstruction in mice
Abstract
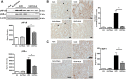
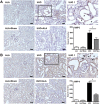
The epithelial-to-mesenchymal transition (EMT) is one of mechanisms that induce renal interstitial fibrosis. Understanding EMT in renal fibrosis has important therapeutic implications for patients with kidney disease. Alpha-lipoic acid (ALA) is a natural compound with antioxidant properties. Studies for ALA are performed in acute kidney injury with renal tubular apoptosis, renal inflammation, and oxidative stress. We investigated the effects of ALA on EMT-mediated renal interstitial fibrosis in mice with unilateral ureteral obstruction (UUO). UUO mice developed severe tubular atrophy and tubulointerstitial fibrosis, with a robust EMT response and ECM deposition after 7 postoperative days. In contrast, ALA-treated UUO mice showed only moderate injury and minimal fibrosis and also larger reductions in the expression of ECM proteins, inflammatory factors, and EMT markers. ALA was shown to be involved in the suppression of infiltrating macrophages associated with EMT and the progression of interstitial fibrosis. It also lessened the destruction of the tubular basement membrane, by reducing the expression of matrix metalloproteinases. This is the first study to show that ALA modulates EMT in a UUO mouse model. Our results suggest that ALA merits further exploration as a therapeutic agent in the prevention and treatment of chronic kidney disease.
Conflict of interest statement
The authors declare no competing financial interests.
Figures




Similar articles
-
Adenovirus-mediated P311 ameliorates renal fibrosis through inhibition of epithelial-mesenchymal transition via TGF-β1-Smad-ILK pathway in unilateral ureteral obstruction rats.Int J Mol Med. 2018 May;41(5):3015-3023. doi: 10.3892/ijmm.2018.3485. Epub 2018 Feb 12. Int J Mol Med. 2018. PMID: 29436600
-
ClC-5 alleviates renal fibrosis in unilateral ureteral obstruction mice.Hum Cell. 2019 Jul;32(3):297-305. doi: 10.1007/s13577-019-00253-5. Epub 2019 May 3. Hum Cell. 2019. PMID: 31054069
-
Alpha1-Antitrypsin Attenuates Renal Fibrosis by Inhibiting TGF-β1-Induced Epithelial Mesenchymal Transition.PLoS One. 2016 Sep 8;11(9):e0162186. doi: 10.1371/journal.pone.0162186. eCollection 2016. PLoS One. 2016. PMID: 27607429 Free PMC article.
-
Unilateral Ureteral Obstruction as a Model to Investigate Fibrosis-Attenuating Treatments.Biomolecules. 2019 Apr 8;9(4):141. doi: 10.3390/biom9040141. Biomolecules. 2019. PMID: 30965656 Free PMC article. Review.
-
Is hydrogen sulfide a potential novel therapy to prevent renal damage during ureteral obstruction?Nitric Oxide. 2018 Feb 28;73:15-21. doi: 10.1016/j.niox.2017.12.004. Epub 2017 Dec 19. Nitric Oxide. 2018. PMID: 29269061 Review.
Cited by
-
Natural Plants Compounds as Modulators of Epithelial-to-Mesenchymal Transition.Front Pharmacol. 2019 Jul 30;10:715. doi: 10.3389/fphar.2019.00715. eCollection 2019. Front Pharmacol. 2019. PMID: 31417401 Free PMC article. Review.
-
Eucalyptol ameliorates Snail1/β-catenin-dependent diabetic disjunction of renal tubular epithelial cells and tubulointerstitial fibrosis.Oncotarget. 2017 Oct 16;8(63):106190-106205. doi: 10.18632/oncotarget.22311. eCollection 2017 Dec 5. Oncotarget. 2017. PMID: 29290941 Free PMC article.
-
Pomolic Acid Ameliorates Fibroblast Activation and Renal Interstitial Fibrosis through Inhibition of SMAD-STAT Signaling Pathways.Molecules. 2018 Sep 3;23(9):2236. doi: 10.3390/molecules23092236. Molecules. 2018. PMID: 30177595 Free PMC article.
-
Cathepsin S regulates renal fibrosis in mouse models of mild and severe hydronephrosis.Mol Med Rep. 2019 Jul;20(1):141-150. doi: 10.3892/mmr.2019.10230. Epub 2019 May 9. Mol Med Rep. 2019. PMID: 31115520 Free PMC article.
-
Alpha-lipoic acid: A promising pharmacotherapy seen through the lens of kidney diseases.Curr Res Pharmacol Drug Discov. 2024 Oct 26;7:100206. doi: 10.1016/j.crphar.2024.100206. eCollection 2024. Curr Res Pharmacol Drug Discov. 2024. PMID: 39524210 Free PMC article. Review.
References
-
- Nath K. A. Tubulointerstitial changes as a major determinant in the progression of renal damage. Am. J. Kidney Dis. 20, 1–17 (1992). - PubMed
-
- Phillips A. O. & Steadman R. Diabetic nephropathy: The central role of renal proximal tubular cells in tubulointerstitial injury. Histol. Histopathol. 17, 247–252 (2002). - PubMed
-
- Eddy A. A. Molecular insights into renal interstitial fibrosis. J. Am. Soc. Nephrol. 7, 2495–2508 (1996). - PubMed
-
- Klahr S., Schreiner G. & Ichikawa I. The progression of renal disease. N. Engl. J. Med. 318, 1657–1666 (1998). - PubMed
-
- Sharma A. K., Mauer S. M., Kim Y. & Michael A. F. Interstitial fibrosis in obstructive nephropathy. Kidney Int. 44, 774–788 (1993). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

