Energy-dense diet triggers changes in gut microbiota, reorganization of gut‑brain vagal communication and increases body fat accumulation
- PMID: 28379213
- PMCID: PMC5382806
- DOI: 10.21307/ane-2017-033
Energy-dense diet triggers changes in gut microbiota, reorganization of gut‑brain vagal communication and increases body fat accumulation
Abstract
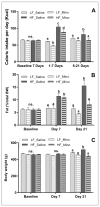
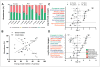
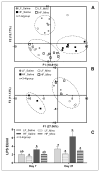
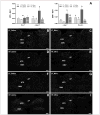
Obesity is associated with consumption of energy-dense diets and development of systemic inflammation. Gut microbiota play a role in energy harvest and inflammation and can influence the change from lean to obese phenotypes. The nucleus of the solitary tract (NTS) is a brain target for gastrointestinal signals modulating satiety and alterations in gut-brain vagal pathway may promote overeating and obesity. Therefore, we tested the hypothesis that high-fat diet‑induced changes in gut microbiota alter vagal gut-brain communication associated with increased body fat accumulation. Sprague-Dawley rats consumed a low energy‑dense rodent diet (LFD; 3.1 kcal/g) or high energy‑dense diet (HFD, 5.24 kcal/g). Minocycline was used to manipulate gut microbiota composition. 16S Sequencing was used to determine microbiota composition. Immunofluorescence against IB4 and Iba1 was used to determine NTS reorganization and microglia activation. Nodose ganglia from LFD rats were isolated and co-cultured with different bacteria strains to determine neurotoxicity. HFD altered gut microbiota with increases in Firmicutes/Bacteriodetes ratio and in pro-inflammatory Proteobacteria proliferation. HFD triggered reorganization of vagal afferents and microglia activation in the NTS, associated with weight gain. Minocycline-treated HFD rats exhibited microbiota profile comparable to LFD animals. Minocycline suppressed HFD‑induced reorganization of vagal afferents and microglia activation in the NTS, and reduced body fat accumulation. Proteobacteria isolated from cecum of HFD rats were toxic to vagal afferent neurons in culture. Our findings show that diet‑induced shift in gut microbiome may disrupt vagal gut‑brain communication resulting in microglia activation and increased body fat accumulation.
Figures
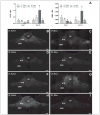

References
-
- Benani A, Hryhorczuk C, Gouaze A, Fioramonti X, Brenachot X, Guissard C, Krezymon A, Duparc T, Colom A, Nedelec E, Rigault C, Lemoine A, Gascuel J, Gerardy-Schahn R, Valet P, Knauf C, Lorsignol A, Penicaud L. Food intake adaptation to dietary fat involves PSA-dependent rewiring of the arcuate melanocortin system in mice. J Neurosci. 2012;32:11970–11979. - PMC - PubMed
-
- Berthoud HR, Carlson NR, Powley TL. Topography of efferent vagal innervation of the rat gastrointestinal tract. Am J Physiol. 1991;260:R200–R207. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

