Intra-endosomal trafficking mediated by lysobisphosphatidic acid contributes to intracellular release of phosphorothioate-modified antisense oligonucleotides
- PMID: 28379543
- PMCID: PMC5605259
- DOI: 10.1093/nar/gkx231
Intra-endosomal trafficking mediated by lysobisphosphatidic acid contributes to intracellular release of phosphorothioate-modified antisense oligonucleotides
Abstract
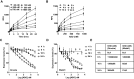
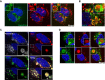
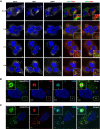
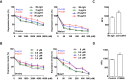
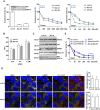
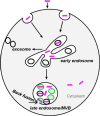
Antisense oligonucleotides (ASOs) with phosphorothioate (PS) linkages are broadly used as research tools and therapeutic agents. Chemically modified PS-ASOs can mediate efficient target reduction by site-specific cleavage of RNA through RNase H1. PS-ASOs are known to be internalized via a number of endocytotic pathways and are released from membrane-enclosed endocytotic organelles, mainly late endosomes (LEs). This study was focused on the details of PS-ASO trafficking through endocytic pathways. It was found that lysobisphosphatidic acid (LBPA) is required for release of PS-ASOs from LEs. PS-ASOs exited early endosomes (EEs) rapidly after internalization and became co-localized with LBPA by 2 hours in LEs. Inside LEs, PS-ASOs and LBPA were co-localized in punctate, dot-like structures, likely intraluminal vesicles (ILVs). Deactivation of LBPA using anti-LBPA antibody significantly decreased PS-ASO activities without affecting total PS-ASO uptake. Reduction of Alix also substantially decreased PS-ASO activities without affecting total PS-ASO uptake. Furthermore, Alix reduction decreased LBPA levels and limited co-localization of LBPA with PS-ASOs at ILVs inside LEs. Thus, the fusion properties of ILVs, which are supported by LBPA, contribute to PS-ASO intracellular release from LEs.
© The Author(s) 2017. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

