AKAP95 interacts with nucleoporin TPR in mitosis and is important for the spindle assembly checkpoint
- PMID: 28379780
- PMCID: PMC5462081
- DOI: 10.1080/15384101.2017.1310350
AKAP95 interacts with nucleoporin TPR in mitosis and is important for the spindle assembly checkpoint
Abstract
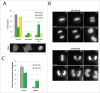
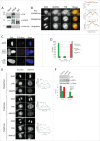
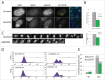
Faithful chromosome segregation during mitosis relies on a proofreading mechanism that monitors proper kinetochore-microtubule attachments. The spindle assembly checkpoint (SAC) is based on the concerted action of numerous components that maintain a repressive signal inhibiting transition into anaphase until all chromosomes are attached. Here we show that A-Kinase Anchoring Protein 95 (AKAP95) is necessary for proper SAC function. AKAP95-depleted HeLa cells show micronuclei formed from lagging chromosomes at mitosis. Using a BioID proximity-based proteomic screen, we identify the nuclear pore complex protein TPR as a novel AKAP95 binding partner. We show interaction between AKAP95 and TPR in mitosis, and an AKAP95-dependent enrichment of TPR in the spindle microtubule area in metaphase, then later in the spindle midzone area. AKAP95-depleted cells display faster prometaphase to anaphase transition, escape from nocodazole-induced mitotic arrest and show a partial delocalization from kinetochores of the SAC component MAD1. Our results demonstrate an involvement of AKAP95 in proper SAC function likely through its interaction with TPR.
Keywords: mitotic arrest deficient; translocated in promoter region.
Figures

Similar articles
-
Nucleoporin translocated promoter region (Tpr) associates with dynein complex, preventing chromosome lagging formation during mitosis.J Biol Chem. 2010 Apr 2;285(14):10841-9. doi: 10.1074/jbc.M110.105890. Epub 2010 Feb 4. J Biol Chem. 2010. PMID: 20133940 Free PMC article.
-
Mps1-mediated release of Mad1 from nuclear pores ensures the fidelity of chromosome segregation.J Cell Biol. 2020 Mar 2;219(3):e201906039. doi: 10.1083/jcb.201906039. J Cell Biol. 2020. PMID: 31913420 Free PMC article.
-
The spindle assembly checkpoint promotes chromosome bi-orientation: A novel Mad1 role in chromosome alignment.Cell Cycle. 2016;15(4):493-7. doi: 10.1080/15384101.2015.1128596. Cell Cycle. 2016. PMID: 26752263 Free PMC article. Review.
-
Therapeutic potential of mitotic interaction between the nucleoporin Tpr and aurora kinase A.Cell Cycle. 2015;14(9):1447-58. doi: 10.1080/15384101.2015.1021518. Cell Cycle. 2015. PMID: 25789545 Free PMC article.
-
Recent Progress on the Localization of the Spindle Assembly Checkpoint Machinery to Kinetochores.Cells. 2019 Mar 23;8(3):278. doi: 10.3390/cells8030278. Cells. 2019. PMID: 30909555 Free PMC article. Review.
Cited by
-
Proximity Labeling Techniques to Study Chromatin.Front Genet. 2020 May 12;11:450. doi: 10.3389/fgene.2020.00450. eCollection 2020. Front Genet. 2020. PMID: 32477404 Free PMC article. Review.
-
The complex activities of the SET1/MLL complex core subunits in development and disease.Biochim Biophys Acta Gene Regul Mech. 2020 Jul;1863(7):194560. doi: 10.1016/j.bbagrm.2020.194560. Epub 2020 Apr 15. Biochim Biophys Acta Gene Regul Mech. 2020. PMID: 32302696 Free PMC article. Review.
-
The autophagy protein Ambra1 regulates gene expression by supporting novel transcriptional complexes.J Biol Chem. 2020 Aug 21;295(34):12045-12057. doi: 10.1074/jbc.RA120.012565. Epub 2020 Jul 2. J Biol Chem. 2020. PMID: 32616651 Free PMC article.
-
Specific inhibition of DPY30 activity by ASH2L-derived peptides suppresses blood cancer cell growth.Exp Cell Res. 2019 Sep 15;382(2):111485. doi: 10.1016/j.yexcr.2019.06.030. Epub 2019 Jun 26. Exp Cell Res. 2019. PMID: 31251903 Free PMC article.
References
-
- Sacristan C, Kops GJPL. Joined at the hip: Kinetochores, microtubules, and spindle assembly checkpoint signaling. Trends Cell Biol 2015; 25:21-8; PMID:25220181; http://dx.doi.org/10.1016/j.tcb.2014.08.006 - DOI - PubMed
-
- Santaguida S, Amon A. Short- and long-term effects of chromosome mis-segregation and aneuploidy. Nat Rev Mol Cell Biol 2015; 16:473-85; PMID:26204159; http://dx.doi.org/10.1038/nrm4025 - DOI - PubMed
-
- Musacchio A. The molecular biology of spindle assembly checkpoint signaling dynamics. Curr Biol 2015; 25:R1002-18; PMID:26485365; http://dx.doi.org/10.1016/j.cub.2015.08.051 - DOI - PubMed
-
- Sironi L, Mapelli M, Knapp S, DeAntoni A, Jeang K-T, Musacchio A. Crystal structure of the tetrameric Mad1-Mad2 core complex: Implications of a “safety belt” binding mechanism for the spindle checkpoint. EMBO J 2002; 21:2496-506; PMID:12006501; http://dx.doi.org/10.1093/emboj/21.10.2496 - DOI - PMC - PubMed
-
- Lee SH, Sterling H, Burlingame A, McCormick F. Tpr directly binds to Mad1 and Mad2 and is important for the Mad1-Mad2-mediated mitotic spindle checkpoint. Genes Dev 2008; 22:2926-31; PMID:18981471; http://dx.doi.org/10.1101/gad.1677208 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
