Application of JC1 for non-toxic isolation of cells with MDR transporter activity by flow cytometry
- PMID: 28380010
- PMCID: PMC5381900
- DOI: 10.1371/journal.pone.0174905
Application of JC1 for non-toxic isolation of cells with MDR transporter activity by flow cytometry
Abstract
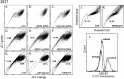
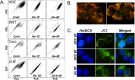
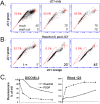
The DNA intercalating dye Hoechst 33342 or its close analog DCV are actively removed from cells by the multidrug resistance transporter ABCG2, a protein overexpressed in metastatic cells and somatic stem cells. In bivariate blue-red flow cytometry fluorescent plots active Hoechst or DCV efflux combined with a concentration dependent bathochromic shifts of these nuclear dyes leads to the segregation of the transporter-rich cells into a distinct cell cohort tilted towards the shorter wavelength axis of the plot, the cohort is generically known as the side population (SP). This feature has facilitated the surface marker-independent isolation of live stem cells. A drawback, though, is the known toxicity of Hoechst dyes. In this study we show that JC1, a bathochromic mitochondrial membrane potential-sensitive dye applied at proper concentration, can yield flow cytometry fluorescent emission bivariate plots containing a low JC1 accumulation (JC1low) cohort. Using a combination of multiple cell lines, ABC-transporter inhibitors and viral vector-driven insertion of the ABCG2 gene or ABCG2 and ABCB1 shRNAs we demonstrate that JC1low can be generated by either of the two aforementioned multidrug resistance transporters. Complete wash out of mitochondrial bound JC1 required more than 24 h. In spite of this tight binding, the dye did not affect either the mitochondrial membrane potentials or the proliferation rate. In contrast, contemporaneous with its nuclear accumulation, Hoechst 33342 or DVC, caused changes in the fluorescent emission of mitochondrial membrane potential sensitive dyes resembling the effects caused by the mitochondrial uncoupler FCCP. In a number of cell lines exposure to Hoechst resulted in marked slow-down of proliferation and abolition of ABCG2 transport activity during the subsequent 2 days but in K562 cells the exposure induced cell extended death. Overall, its lack of toxicity vis. a vis. the toxicity and genotoxicity of the DNA intercalating dyes makes JC1 an ideal tool for isolating live cells expressing high multidrug resistance transport activity.
Conflict of interest statement
Figures






Similar articles
-
The multidrug resistance transporter ABCG2 (breast cancer resistance protein 1) effluxes Hoechst 33342 and is overexpressed in hematopoietic stem cells.Clin Cancer Res. 2002 Jan;8(1):22-8. Clin Cancer Res. 2002. PMID: 11801536
-
ABCG2-dependent dye exclusion activity and clonal potential in epithelial cells continuously growing for 1 month from limbal explants.Invest Ophthalmol Vis Sci. 2011 Jun 17;52(7):4330-7. doi: 10.1167/iovs.10-5897. Invest Ophthalmol Vis Sci. 2011. PMID: 21421882 Free PMC article.
-
High-throughput flow cytometry to detect selective inhibitors of ABCB1, ABCC1, and ABCG2 transporters.Assay Drug Dev Technol. 2008 Apr;6(2):263-76. doi: 10.1089/adt.2007.107. Assay Drug Dev Technol. 2008. PMID: 18205550
-
ABCG2 (BCRP) expression in normal and malignant hematopoietic cells.Hematol Oncol. 2003 Sep;21(3):115-30. doi: 10.1002/hon.714. Hematol Oncol. 2003. PMID: 14579240 Review.
-
Flow cytometry of the side population (SP).Curr Protoc Cytom. 2013;Chapter 9:9.23.1-9.23.20. doi: 10.1002/0471142956.cy0923s64. Curr Protoc Cytom. 2013. PMID: 23546779 Review.
Cited by
-
Immunomodulatory Effects of Bacterial Toll-like Receptor Ligands on the Phenotype and Function of Milk Immune Cells in Dromedary Camel.Biology (Basel). 2023 Feb 9;12(2):276. doi: 10.3390/biology12020276. Biology (Basel). 2023. PMID: 36829554 Free PMC article.
-
The Impact of Anticoagulation Agent on the Composition and Phenotype of Blood Leukocytes in Dromedary Camels.Vet Sci. 2022 Feb 13;9(2):78. doi: 10.3390/vetsci9020078. Vet Sci. 2022. PMID: 35202331 Free PMC article.
-
Immunomodulatory Effects of the Cyclooxygenase Inhibitor Lornoxicam on Phenotype and Function of Camel Blood Leukocytes.Animals (Basel). 2021 Jul 6;11(7):2023. doi: 10.3390/ani11072023. Animals (Basel). 2021. PMID: 34359151 Free PMC article.
-
Clinical and biological impact of ATP-binding cassette transporter activity in adult acute myeloid leukemia.Haematologica. 2023 Jan 1;108(1):61-68. doi: 10.3324/haematol.2022.280676. Haematologica. 2023. PMID: 35924580 Free PMC article.
-
Changes in Cell Vitality, Phenotype, and Function of Dromedary Camel Leukocytes After Whole Blood Exposure to Heat Stress in vitro.Front Vet Sci. 2021 Apr 9;8:647609. doi: 10.3389/fvets.2021.647609. eCollection 2021. Front Vet Sci. 2021. PMID: 33898545 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

