Comparison of the current AJCC-TNM numeric-based with a new anatomical location-based lymph node staging system for gastric cancer: A western experience
- PMID: 28380037
- PMCID: PMC5381862
- DOI: 10.1371/journal.pone.0173619
Comparison of the current AJCC-TNM numeric-based with a new anatomical location-based lymph node staging system for gastric cancer: A western experience
Abstract
Background: In gastric cancer, the current AJCC numeric-based lymph node staging does not provide information on the anatomical extent of the disease and lymphadenectomy. A new anatomical location-based node staging, proposed by Choi, has shown better prognostic performance, thus soliciting Western world validation.
Study design: Data from 284 gastric cancers undergoing radical surgery at the Second University of Naples from 2000 to 2014 were reviewed. The lymph nodes were reclassified into three groups (lesser and greater curvature, and extraperigastric nodes); presence of any metastatic lymph node in a given group was considered positive, prompting a new N and TNM stage classification. Receiver-operating-characteristic (ROC) curves for censored survival data and bootstrap methods were used to compare the capability of the two models to predict tumor recurrence.
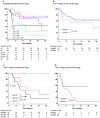
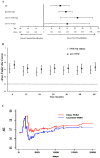
Results: More than one third of node positive patients were reclassified into different N and TNM stages by the new system. Compared to the current staging system, the new classification significantly correlated with tumor recurrence rates and displayed improved indices of prognostic performance, such as the Bayesian information criterion and the Harrell C-index. Higher values at survival ROC analysis demonstrated a significantly better stratification of patients by the new system, mostly in the early phase of the follow-up, with a worse prognosis in more advanced new N stages, despite the same current N stage.
Conclusions: This study suggests that the anatomical location-based classification of lymph node metastasis may be an important tool for gastric cancer prognosis and should be considered for future revision of the TNM staging system.
Conflict of interest statement
Figures

References
-
- Lowenfeld L, Datta J, Lewis RS Jr, McMillan MT, Mamtani R, Damjanov N, et al. Multimodality Treatment of T4 Gastric Cancer in the United States: Utilization Trends and Impact on Survival. Ann Surg Oncol. 2015;22: S863–S872 (Suppl 3). - PubMed
-
- Reim D, Loos M, Vogl F, Novotny A, Schuster T, Langer R, et al. Prognostic implications of the seventh edition of the international union against cancer classification for patients with gastric cancer: the Western experience of patients treated in a single-center European institution. J Clin Oncol. 2013;31: 263–271. 10.1200/JCO.2012.44.4315 - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

