Biodegradable poly (lactic acid-co-glycolic acid) scaffolds as carriers for genetically-modified fibroblasts
- PMID: 28380080
- PMCID: PMC5381796
- DOI: 10.1371/journal.pone.0174860
Biodegradable poly (lactic acid-co-glycolic acid) scaffolds as carriers for genetically-modified fibroblasts
Abstract
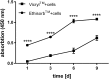
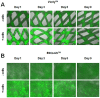
Recent advances in gene delivery into cells allow improved therapeutic effects in gene therapy trials. To increase the bioavailability of applied cells, it is of great interest that transfected cells remain at the application site and systemic spread is minimized. In this study, we tested clinically used biodegradable poly(lactic acid-co-glycolic acid) (PLGA) scaffolds (Vicryl & Ethisorb) as transient carriers for genetically modified cells. To this aim, we used human fibroblasts and examined attachment and proliferation of untransfected cells on the scaffolds in vitro, as well as the mechanical properties of the scaffolds at four time points (1, 3, 6 and 9 days) of cultivation. Furthermore, the adherence of cells transfected with green fluorescent protein (GFP) and vascular endothelial growth factor (VEGF165) and also VEGF165 protein secretion were investigated. Our results show that human fibroblasts adhere on both types of PLGA scaffolds. However, proliferation and transgene expression capacity were higher on Ethisorb scaffolds most probably due to a different architecture of the scaffold. Additionally, cultivation of the cells on the scaffolds did not alter their biomechanical properties. The results of this investigation could be potentially exploited in therapeutic regiments with areal delivery of transiently transfected cells and may open the way for a variety of applications of cell-based gene therapy, tissue engineering and regenerative medicine.
Conflict of interest statement
Figures




Similar articles
-
Hyaluronic acid/poly(lactic-co-glycolic acid) core/shell fiber meshes loaded with epigallocatechin-3-O-gallate as skin tissue engineering scaffolds.J Nanosci Nanotechnol. 2014 Nov;14(11):8458-63. doi: 10.1166/jnn.2014.9922. J Nanosci Nanotechnol. 2014. PMID: 25958546
-
Fabrication of poly(lactic-co-glycolic acid) scaffolds containing silk fibroin scaffolds for tissue engineering applications.J Biomed Mater Res A. 2014 Aug;102(8):2713-24. doi: 10.1002/jbm.a.34947. Epub 2013 Sep 24. J Biomed Mater Res A. 2014. PMID: 24026912
-
Effect of fiber orientation of collagen-based electrospun meshes on human fibroblasts for ligament tissue engineering applications.J Biomed Mater Res B Appl Biomater. 2015 Jan;103(1):39-46. doi: 10.1002/jbm.b.33153. Epub 2014 Apr 23. J Biomed Mater Res B Appl Biomater. 2015. PMID: 24757041 Free PMC article.
-
A dual-application poly (dl-lactic-co-glycolic) acid (PLGA)-chitosan composite scaffold for potential use in bone tissue engineering.J Biomater Sci Polym Ed. 2017 Nov;28(16):1966-1983. doi: 10.1080/09205063.2017.1364100. Epub 2017 Aug 17. J Biomater Sci Polym Ed. 2017. PMID: 28777694
-
Importance of Poly(lactic-co-glycolic acid) in Scaffolds for Guided Bone Regeneration: A Focused Review.J Oral Implantol. 2015 Aug;41(4):e152-7. doi: 10.1563/AAID-JOI-D-13-00225. Epub 2014 Feb 19. J Oral Implantol. 2015. PMID: 24552153 Review.
Cited by
-
Nucleic acid direct delivery to fibroblasts: a review of nucleofection and applications.J Biol Eng. 2022 Nov 4;16(1):30. doi: 10.1186/s13036-022-00309-5. J Biol Eng. 2022. PMID: 36329479 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

